PEDIATRICS: DISORDERS OF SEXUAL DIFFERENTIATION
Congenital Adrenal Hyperplasia
Normal Sexual Differentiation
- Normal Genotypic Development
- Chromosomal Sex
- SRY (sex-determining region Y gene)
- An evolutionarily conserved gene on the Y chromosome of mammals
- On the short arm of the Y chromosome near the centromere, adjacent to the pseudoautosomal boundary
- Specifies development of the testis (testis-determining factor); responsible for maleness
- SRY protein functions:
- A transcription factor that, by binding and producing bending of the DNA, promotes protein-protein interaction and activates downstream gene expression
- SRY protein has a characteristic high-mobility group (HMG), DNA-binding domain that can induce significant DNA binding when bound to the regulatory regions of target genes
- Supporting cells of the developing male urogenital ridge.
- These cells participate in cord formation and ultimately develop into Sertoli cells.
- A transcription factor that, by binding and producing bending of the DNA, promotes protein-protein interaction and activates downstream gene expression
- SRY expression leads to induction of SOX (SRY box-related) 9, a subfamily of closely related genes, during gonadal differentiation
- Additional Genes Involved in Gonadal Determination
- Duplication of an X-specific gene causes XY sex reversal by expressing a double dose of a region normally subject to X inactivation. This region is designated as the dosage-sensitive sex (DSS) reversal critical region
- Normal Phenotypic Development
- Gonadal Stage of Differentiation
- During the first 6 weeks of embryonic development, the gonoads (gonadal ridge, germ cells, internal ducts, and external genitalia) are bipotential in both 46,XY and 46,XX embryos
- Posterior to the degenerating pronephros (primary kidney), the mesonephric/wolffian duct develops and extends in an anteroposterior direction.
- The metanephros arises from inductive interactions between the ureteric bud that branches from the caudal wolffian duct and mesenchyme.
- Soon after formation of the wolffian duct, the paramesonephric/mullerian duct grows rostral to caudal, adjacent lateral the wolffian duct until the duct joins at the urogenital sinus.
- Under the genetic influences of SRY, the bipotential gonadal ridges differentiate into either ovaries or testes, and germ cells develop into either oocytes or spermatocytes.
- In the absence of SRY, ovarian organogenesis results.
- Duplicate copies of at least one X-chromosomal locus are necessary (which presumably explains the dysgenetic ovaries in the 45,XO Turner syndrome patients) for development of the ovary.
- The precise moment at which this occurs remains unknown.
- In the absence of SRY, ovarian organogenesis results.
- In response to SRY, differentiation of Sertoli cells is noted at 6-7 weeks’ gestation.
- Germ cells reach a maximum endowment of 20 million cells by 20 weeks’ gestation
- Male development
- The initial endocrine function of the fetal testes is the secretion of Anti-Mullerian Hormone (AMH)/Mullerian Inhibiting Substance (MIS) by the Sertoli cells at 7 to 8 weeks’ gestation.
- AMH/MIS
- One of the two hormones (androgens being the other) necessary for male sexual differentiation
- Member of the transforming growth factor-β (TGF-β) family
- Acts locally and unilaterally to suppress the müllerian ducts
- Masculinization of the male fetus starts at 7 to 8 weeks of gestation; before the 8th week of gestation the urogenital tract is identical in the two sexes; the first sign of male phenotypic differentiation is degeneration of the müllerian ducts adjacent to the testes
- By 10 weeks of gestation, degeneration of the müllerian ducts is almost complete and the wolffian ducts have become more prominent
- Müllerian duct remnants in the male (2):
- Appendix testis: a small tissue protrusion at the superior pole of the testicle
- Prostatic utricle: the posterior expansion of the prostatic urethra
- The verumontanum or seminal colliculus is the rounded eminence of the urethral crest within the posterior wall of the mid prostatic urethra. The prostatic utricle opens into it in the midline and the two ejaculatory ducts open just distal to the utricle
- Present in 10-15% of boys with a proximal hypospadias
- Most common cause of difficulty in catheterizing the bladder with proximal hypospadias
Prostatic urethra anatomy.
Source: Wikipedia
- Androgens
- Leydig cells in the interstitium differentiate at 8-9 weeks
- Testosterone secretion is detectable at ≈9 weeks’ gestation
- Initially, androgens are synthesized by the Leydig cells autonomously.
- Later, synthesis is dependent on placental human chorionic gonadotropin (hCG) secretion.
- Later in gestation, with declining hCG concentrations, androgen synthesis is dependent on luteinizing hormone (LH) secretion by the fetal pituitary gland.
- There is a rise in serum and testicular testosterone to a peak concentration at 13 weeks and then a decline
- The rate-limiting enzyme for fetal testosterone synthesis is 3β-hydroxysteroid dehydrogenase, which is concentrated ≈50x more highly in the fetal testes than in the ovary.
- Androgens are essential for virilization of (3):
- Wolffian duct structures
- Urogenital sinus
- Genital tubercle
- Male structures derived from wolffian ducts (5):
- Body and tail of epididymis
- Efferent ductules and head of epididymis are derived from the mesonephric tubules
- Vas deferens
- Seminal vesicles
- Distally, the wolffian ducts join the urogenital sinus by about 30 days gestation, where they develop into the seminal vesicles
- Ejaculatory duct
- Appendix epididymis
- Body and tail of epididymis
- Male structures derived from urogenital sinus (3):
- Prostate
- Bulbourethral glands
- Prostatic and Penile Urethra§
- Male structures derived from genital tubercle (1):
- Penis
- Male structures derived from wolffian ducts (5):
- Testosterone
- The major androgen secreted by the testes, enters target tissues by passive diffusion
- The local source of androgen is important for ipsilateral Wolffian duct development, which does not occur if testosterone is supplied only via the peripheral circulation
- [Clinical implication: if androgen synthesis is deficient on one side due to testicular dysfunction (e.g. agenesis), the ipsilateral structures derived from the Wollfian duct (see above) may be not develop normally]
- Dihydrotestosterone (DHT)
- Testosterone is converted to DHT by intracellular 5α-reductase
- DHT binds to the androgen receptor with greater affinity and stability than does testosterone.
- 5α-reductase is found in the prostate, urogenital sinus, and external genitalia
- In tissues with 5α-reductase at the time of sexual differentiation, DHT is the active androgen
- The primary enzyme in the prostate is 5α-reductase, type 2. A deletion in the gene coding for this enzyme has been discovered in patients with 5α-reductase deficiency
- The prostate gland begins to develop during the 10-12th week.
- Similar to renal and bladder development, prostatic development depends on mesenchymal-epithelial interactions but under the influence of androgens.
- There is no evidence that AMH/MIS plays a direct role in prostate development
- At 9-13 weeks of gestation, dihydrotestosterone (DHT) simulates androgen receptors the
- Genital tubercle to differentiate into penis
- Even after the phallus is developed, the term genital tubercle remains, but only as the terminal end of it, which develops into either the glans penis.§
- Urethral folds to differentiate into the corpus spongiosum/penile shaft
- The urogenital sinus extends between the urethral folds and forms the urethral groove, which is lined with endoderm.
- Labioscrotal swellings to differentiate into scrotum
- Also known as genital swellings or labioscrotal folds§
- By 12 to 13 weeks’ gestation, the genitalia of the male fetus are completed
- Under the influence of androgen secreted by the fetal testes, penile growth and testicular descent occur in the third trimester
- Female development
- Testosterone is not secreted by the ovaries and therefore the Wolffian ducts regress
- Wolffian duct remnants in the female (2):
- Gartner duct
- Can form cysts near the vaginal introitus and anterolateral vaginal wall
- Epoophoron and paroophoron: in the mesentery of the ovary
- Gartner duct
- Wolffian duct remnants in the female (2):
- Estrogen synthesis by the ovary is detectable in the female embryo just after 8 weeks of gestation.
- The rate-limiting enzyme is aromatase, which is higher in the fetal ovary than in the fetal testis.
- Estrogens are not required for normal female differentiation of the reproductive tract, but they can interfere with male differentiation.
- Estrogen can block the effect of AMH/MIS on Müllerian ducts
- Prenatal estrogen treatment of mothers has been associated with male reproductive tract abnormalities
- Because the ovary does not produce AMH/MIS, the Müllerian ducts are maintained and develop into the female internal reproductive tract (4):
- Fallopian tubes
- Uterus
- Cervix
- Proximal 2/3rd of the vagina
- The distal 1/3rd of the vagina is derived from the urogenital sinus
- In females and males with abnormalities in testosterone and/or DHT production, 5α-reductase deficiency, or androgen-receptor insufficiency, the primitive perineum does not lengthen and the
- Genital tubercle bends inferiorly, passively become the clitoris
- Urethral folds passively become the labia minora
- Labioscrotal swellings passively become the labia majora
- The definitive urogenital sinus becomes the vestibule of the vagina
- 5α-reductase deficiency more likely associated with ambiguous genitalia whereas complete androgen-receptor insufficiency have near-normal female external phenotype
- Gender Identity, Gender Role, and Gender Orientation
- The previously accepted dogma that children are psychosexually neutral at birth and capable of being environmentally oriented (the blue room/pink room theory) has been challenged by those who support the concept of prenatal psychosexual differentiation
UrologySchool.com Summary of Embyrologic Origins of the GU Tract
| Common§ | Males | Females | |
| Urogenital sinus | Bladder | Transitional and peripheral zone of prostate§ Prostatic and penile urethra Bulbourethral glands |
Distal 1/3 of vagina Urethra |
| Wollfian duct | Renal collecting tubules and ducts, calyces, infundibulae, pelvis, and ureters§§ | Central zone of prostate§ Body and tail of epididymis Vas deferens Seminal vesicles Ejaculatory duct Appendix epididymis |
Remnants: Gartner duct Epoopheron or paraoopheron |
| Mullerian duct | Remnants: Appendix testis Prostatic utricle |
Fallopian tubes Uterus Cervix Proximal 2/3rd of the vagina |
|
| Mesonephric tubules | Efferent ductules and head of epididymis | ||
| Genital tubercle | Penis§ | Clitoris | |
| Urethral folds | Corpus spongiosum/penile shaft | Labia minora | |
| Labioscrotal swellings | Scrotum | Labia majora |
Abnormal Sexual Differentiation
- Categories (5):
- Disorders of gonadal differentiation and development
- Ovotesticular DSD
- 46,XX DSD (the over-masculinized female; ovaries present but external genitalia exhibiting evidence of masculinization)
- 46,XY DSD (the under-masculinized male; testes present but genital ducts and/or external genitalia incompletely masculinized)
- Unclassified forms
- If a DSD is discovered, patients should be followed in a multidisciplinary clinic specific to these complex diagnoses.
Disorders of Gonadal Differentiation and Development
- Includes (6):
- Klinefelter Syndrome
- 46XX Males
- Turner Syndrome
- 46XX Pure Gonadal Dysgenesis
- Mixed Gonadal Dysgenesis
- 46XY Partial Gonadal Dysgenesis
- Klinefelter syndrome
- Karyotype: characterized by 1 Y chromosome and ≥ 2 X chromosomes
- Most common karyotype will be 47,XXY
- Epidemiology
- Incidence: 1 in 600 live-born males
- Most common major abnormality of sexual differentiation
- Incidence: 1 in 600 live-born males
- Clinical characteristics (6):
- Small, firm testes
- Seminiferous tubules undergo replacement with hyaline after pubertal development
- Predisposed to developing extra-gonadal (e.g. mediastinal) germ cell tumours as well as non-germ cell tumours (Leydig and Sertoli cell tumors [not gonadoblastoma])
- Routine surveillance scrotal ultrasonography has been advocated for post-pubertal patients
- Primary hypogonadism
- Leydig cells are present, but testosterone production is abnormally low, with elevated gonadotropins and estradiol
- The decreased androgen production may impair normal secondary sexual development.
- Muscle development may be poor, and the fat distribution is more female than male
- Facial hair is sparse
- Gynecomastia
- Common development during puberty
- Result of an increased ratio of estradiol to testosterone
- Can be quite marked
- 8x the risk for development of breast carcinoma, requiring lifelong surveillance after puberty
- Azoospermia
- Vast majority are azoospermic
- Presence of sperm suggests 46,XY/47,XXY mosaicism
- Fertility is possible with the use of testicular sperm extraction (TESE)
- Some advocate combining intracytoplasmic sperm injection (ICSI) with pre-implantation diagnosis, given the lower (54%) rate of normal embryos from Klinefelter syndrome patients
- Vast majority are azoospermic
- Eunuchoidism
- Above average height, mainly because of the disproportionate length of their legs
- Neurophysiologic and cognitive deficits
- Decreased verbal skills, frontal executive function and cognitive skills
- Small, firm testes
- Management
- Androgen supplementation in selected male patients to improve libido
- Reduction mammoplasty, if necessary
- Surveillance for testicular tumor and breast carcinoma
- Karyotype: characterized by 1 Y chromosome and ≥ 2 X chromosomes
- 46,XX Males
- Karyotype: 46XX
- Characterized by testicular development in subjects who have two X chromosomes and lack a normal Y chromosome
- Two categories of patients with XX maleness have been identified:
- SRY positive (90% of cases)
- The most common mechanism to explain sex reversal is translocation of Y-chromosomal material, including SRY, to the X chromosome.
- Rarely have genital abnormalities, but they have phenotypic features of Klinefelter syndrome, including hypogonadism, gynecomastia, azoospermia. These patients differ from those with Klinefelter syndrome in that they are shorter and have normal skeletal proportions
- SRY negative
- More commonly have genital ambiguity
- Clinical characteristics:
- Most of these subjects have normal male external genitalia, but 10% have hypospadias
- All are infertile
- Management
- Similar to Klinefelter syndrome
- Androgen replacement benefits selected patients, and reduction mammoplasty may be beneficial.
- Likely that these patients will also be at increased risk for breast carcinoma and testis tumor.
- Unable to have biological children because of their lack of germ cell elements
- Those classic patients with infertility would not benefit from testicular biopsy for potential intracytoplasmic sperm injection
- Turner syndrome
- Karyotype: 45X
- Presence of only one normally functioning X chromosome; the other sex chromosome may be absent or abnormal, or mosaicism may be present
- Clinical characteristics:
- Female phenotype
- Short stature
- Lymphedema
- Majority of the associated congenital anomalies can be explained by the presence of lymphedema at critical points in development, leading to an imbalance in growth forces
- Broad chest, webbed neck, widespread nipples, cubitus valgus (increased carrying angle at the elbows), peripheral edema at birth, short fourth metacarpal, hypoplastic nails, multiple pigmented nevi
- Primary amenorrhea
- Common cause of primary amenorrhea, and the diagnosis is frequently made because pubertal development never occurs
- Lack of secondary sexual characteristics
- Pubic and axillary hair fails to develop in normal abundance, and the well-differentiated external genitalia, vagina, müllerian derivatives, and breasts remain small
- Gonadal dysgenesis
- Ovaries become streaks and are located in the broad ligament
- Histologically, the hypoplastic streak possesses interlacing waves of dense fibrous stroma that is devoid of oocytes but is otherwise indistinguishable from normal ovarian stroma.
- Both estrogen and androgen are decreased, and levels of FSH and LH are increased
- Coarctation of the aorta, bicuspid aortic valve, and renal anomalies
- 33-60% have structural or positional abnormalities of the kidney such as horseshoe kidney, duplication or renal agenesis, and malrotation
- Neurophysiologic and cognitive deficits
- Differences in parietal and temporal lobe anatomy and posterior fossa morphology
- Diagnosis and Evaluation
- May be diagnosed prenatally on the basis of a variety of ultrasound findings (increased nuchal translucency, lymphedema, cystic hygroma, coarctation of the aorta, renal anomalies) or by abnormal results of fetal karyotyping.
- Management
- Must identify possible Y-chromosomal material or 45X/46XY mosaicism
- Occult Y-chromosomal material in the neonate is evaluated with fluorescence in situ hybridization (FISH) or PCR
- Risk of gonadoblastoma (in situ germ cell cancer of low malignant potential) with occult Y-chromosomal material: 12%
- Gonadoblastoma is associated with dysgerminoma or other germ cell neoplasms
- Because the age of occurrence of gonadoblastoma is variable and has been reported as early as age 10 months, timely prophylactic excision of the streak gonads in the Y mosaic Turner syndrome patient is advised
- Streak gonads in confirmed 45,XO patients (without any Y-chromosome material) do not need to be removed
- Ultrasound screening for renal and cardiac abnormalities
- Human growth hormone has successfully been used in children to achieve increased adult height.
- At an appropriate age, typically 12-15 years, exogenous hormonal therapy to induce puberty and then to maintain a normal female endocrine status is begun.
- A spectrum of potential gonadal function has been noted in large series of patients with Turner syndrome. In 2-5% of Turner patients, spontaneous menses will occur with a potential to achieve pregnancy independently, although spontaneous fertility is rare.
- Because of a high likelihood of premature ovarian failure, early oocyte preservation may be useful for long-term fertility preservation.
- Turner syndrome patients are at increased risk of bladder and urethral cancer
- 46,XX “Pure” Gonadal Dysgenesis
- Karyotype: 46XX
- Condition entails gonadal dysgenesis only; these patients exhibit none of the somatic stigmata associated with Turner syndrome
- Clinical characteristics:
- Bilateral streak gonads
- Sexual infantilism
- Normal female external genitalia
- Normal müllerian ducts with absence of wolffian duct structures
- Normal height
- The streak gonads result in elevated serum gonadotropins
- Management
- Proper cyclic hormone replacement with estrogen and progesterone.
- In contrast to Turner syndrome, growth is not abnormal with this condition, and therefore growth hormone should not be required.
- Because, by definition, these patients have no Y-chromosomal material, gonadectomy is not required.
- Mixed Gonadal Dysgenesis (MGD)
- Karyotype: most 45,XO/46,XY
- Clinical characteristics (4):
- Unilateral dysgenetic testis, which is often intra-abdominal
- Contralateral streak gonad
- Persistent contralateral müllerian structures
- Varying degrees of inadequate masculinization
- The phenotypic spectrum of patients with XO/XY mosaicism ranges from phenotypic females with Turner syndrome, to those with ambiguous genitalia, to those with normal male genitalia.
- MGD is the 2nd most common cause of ambiguous genitalia (after CAH) in the neonatal period and must be in the differential diagnosis.
- The majority of these patients have varying degrees of phallic development, a urogenital sinus with labioscrotal fusion, and an undescended testis.
- The dysgenetic or streak gonad is associated with ipsilateral müllerian derivatives (uterus, fallopian tube)
- In virtually all patients, a uterus, vagina, and fallopian tube are present.
- The dysgenetic testis
- Is capable of responding to gonadotropins and secreting testosterone in normal quantities at puberty
- Lacks germinal elements, so infertility is the rule
- Increased risk of developing
- A gonadal tumor (gonadoblastoma, dysgerminoma)
- Gonadoblastoma is the most common
- Wilms tumor
- Management
- Gender assignment
- Appropriate gonadectomy
- Proper screening for Wilms tumor
- Partial Gonadal Dysgenesis.
- Karyotype: typically 45,X/46,XY or 46,XY
- Characterized by two dysgenetic testes
- Closely related to mixed gonadal dysgenesis (one dysgenetic testis and a streak gonad)
- Clinical characteristics:
- Spectrum of external genital abnormalities
- Depends on the capability of the dysgenetic gonads to produce testosterone
- Persistent müllerian structures
- Typically present, but to varying degrees depending on MIS secretion by the dysgenetic gonads
- Increased risk for gonadal (gonadoblastoma, dysgerminoma) malignancy
- Management
- Similar to mixed gonadal dysgenesis
- 46,XY Complete (“Pure”) Gonadal Dysgenesis (Swyer Syndrome)
- Karyotype: may be due to an abnormality of the SRY gene
- Mutations in the SRY gene are the cause in 10-15% of cases
- Characterized by:
- [Female phenotype] normal female genitalia, well-developed müllerian structures
- Bilateral streak gonads
- Non-mosaic karyotype
- Because there is complete absence of testicular determination in this condition, ambiguity of genitalia is not an issue, but sexual infantilism is the primary clinical problem
- Majority present in their teens with delayed puberty and amenorrhea
- Increased risk for germ cell tumors
- Gonadoblastoma is most common, and it is frequently bilateral
- Management:
- Removal of both streak gonads and proper cyclic hormone replacement with estrogen and progesterone.
Ovotesticular Disorder of Sex Development
- Karyotype: 46,XX though many patients will have a second mosaic cell line with a Y-chromosome present
- Characterized by presence of both testicular tissue with well-developed seminiferous tubules and ovarian tissue with primordial follicles
- May take the form of one ovary and one testis or, more commonly, one or two ovotestes
- Both the external genitalia and internal duct structures of ovotesticular DSD display gradations between male and female
- In most patients the external genitalia are ambiguous but masculinized to variable degrees
- Differentiation of the internal ducts is related to the function of the ipsilateral gonad.
- Fallopian tubes (Mullerian structure) are consistently present on the side of the ovary, and a vas deferens (Wolffian structure) is always present adjacent to a testis.
- The ovotestis, which comprises two thirds of gonads in ovotesticular DSD, is associated with a fallopian tube in two thirds of patients and with either a vas deferens only or both structures in one third of patients.
- Most patients have a uterus
- Can have cyclical hematuria§
- Management
- Gender assignment based on the potential for fertility is the most important aspect of management
- Histopathology of the ovotestis will typically demonstrate well-developed ovarian tissue and a dysgenetic testicular component
- Unlike patients with most other forms of gonadal dysgenesis, individuals with ovotesticular DSD the and the appropriate ductal structures have the potential for fertility if raised as female.
- Ovulation and pregnancy have been reported for female patients with 46,XX ovotesticular DSD
- Male fertility has not been clearly documented
- If the patient is to be raised as female, all testicular and wolffian-derived tissue should be removed.
- Postoperative stimulation with hCG to confirm that all testicular tissue has been removed is recommended.
- When ovarian tissue is preserved, normal ovarian function can occur at puberty, although hormonal replacement may be necessary.
- Careful surveillance for potential gonadal tumors in the patient raised as female is also advisable.
- If a male gender is assigned, as has been most common historically, all ovarian and müllerian tissue should be removed.
- 75% are raised as male
- Consideration should be given to gonadectomy at puberty with appropriate androgen replacement given the high risk of malignancy and unlikelihood of male fertility
- Gender assignment based on the potential for fertility is the most important aspect of management
46,XX Disorder of Sex Development (Over-masculinized Female)
- 46,XX individuals with ovaries have a partially masculinized phenotype and ambiguous genitalia
- Causes (3):
- Congenital Adrenal Hyperplasia
- Most common cause of the masculinized female
- Most common cause of ambiguous genitalia in the newborn
- Maternal ingestion of androgens (very rare)
- Virilizing tumours in the mother (very rare)
- Because gonadal females have androgen receptor within their tissues, exogenous androgen produces virilization
- The degree to which any androgen or progestational agent affects female fetal development is a function of the strength of the agent, its maternal dosage, and timing and duration of administration
- Congenital Adrenal Hyperplasia
- Congenital Adrenal Hyperplasia
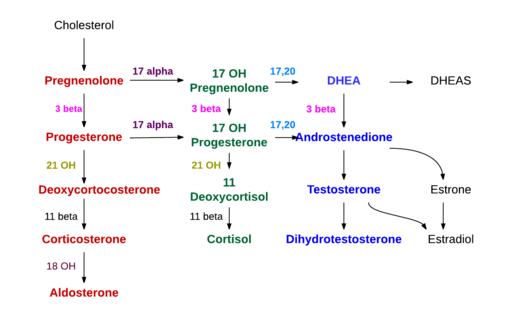
- Pathogenesis
- Classic example of an inborn error of metabolism—in this case, an error involving cortisol synthesis
- A defect in any of the enzymes involved in the steroidogenesis pathway may result in CAH
- 21-hydroxylase deficiency
- Autosomal recessive inheritance
- Most common (95%) cause of CAH
- 11β-hydroxylase deficiency
- Second most common (5%) cause of CA
- 3β-hydroxysteroid dehydrogenase deficiency
- Least common enzyme deficiency responsible for a virilizing form of CAH
- Deficiencies in 21-hydroxylase or 11β-hydroxylase results in impaired formation of cortisol, causing a compensatory increase in the secretion of ACTH. This increased ACTH enhances formation of adrenal steroids proximal to the enzymatic defect and a secondary increase in the formation of testosterone, the active androgen in CAH.
- 21-hydroxylase deficiency
Steroidogenesis
Source: Wikipedia
- Categorized clinically (3):
- Classic salt wasters: virilization and aldosterone deficiency
- Simple virilizers: virilization without aldosterone deficiency
- Non-classic patients: neither virilization nor aldosterone deficiency
- Wide clinical spectrum likely due to different degrees of enzymatic dysfunction conferred by specific, identifiable genetic defects.
- The severity of the virilization is usually greater in infants who experience salt wasting
- Classic salt wasters
- In the female with the classic salt-wasting and simple virilizing forms of the disorder, a masculinized female results
- Because impaired steroidogenesis begins early in life—at the time of formation of the external genitalia (beginning at 10 weeks’ gestation)— there is virtually always evidence of some degree of masculinization at birth. This manifests as enlargement of the clitoris and varying degrees of labial fusion
- In both males and females, symptoms related to salt-deficiency and hypocortisolism begin within the first few weeks after birth, with failure to regain birth weight, progressive weight loss, and dehydration.
- In severely affected infants, adrenal crises occur within the first 10 to 21 days of life.
- Vomiting is prominent and can be so extreme that a mistaken diagnosis of pyloric stenosis is made, particularly in the male.
- In the female with the classic salt-wasting and simple virilizing forms of the disorder, a masculinized female results
- Simple virilizers
- In males with the simple-virilizer form, the main clinical manifestations are those of isosexual precocity
- The infant appears normal at birth, but signs of sexual and somatic precocity appear within the first 2-3 years of life.
- Enlargement of the penis, scrotum, and prostate occur, accompanied by the appearance of pubic hair, acne, and deepening of the voice
- Musculature is well developed (prompting the descriptive term “little Hercules”), and bone age is more advanced than appropriate for the chronologic age.
- Testes remain normal in size
- Two major long-term implications are short stature as well as infertility
- Infertility is often related to the finding of testicular adrenal rest nodules
- Adrenal rest nodules are most reliably diagnosed with scrotal ultrasonography
- Treatment may involve intensifying glucocorticoid treatment and testis-sparing excision
- Infertility is often related to the finding of testicular adrenal rest nodules
- The infant appears normal at birth, but signs of sexual and somatic precocity appear within the first 2-3 years of life.
- In males with the simple-virilizer form, the main clinical manifestations are those of isosexual precocity
- 21-hydroxylase deficiency
-
Patients with 21-hydroxylase deficiency usually have either the classic salt wasting (75%) or simple virilization (25%) form
- Mutations leading to gene conversion of the active CYP21A gene into the inactive gene occur in 65-90% of cases of the classic disorder (salt wasting and simple virilizing) and all cases of non-classic 21-hydroxylase deficiency
- Non-classic 21-hydroxylase deficiency
- Represents an attenuated, late-onset form that is variable in its clinical severity because of partial deficiency of 21-hydroxylase and timing of onset
- Incidence:1 in 100
- Most common autosomal recessive disorder in humans
- Patients do not have cortisol deficiency but do have hyperandrogenism [less severe than virilization?]
- The presenting symptoms in females are commonly hirsutism and oligomenorrhea, male pattern baldness, and polycystic ovaries.
- Diagnosis and Evaluation
- History and Physical Exam
- Radioimmunoassay
for 17-hydroxyprogesterone
- Has replaced the more cumbersome 24-hour urine collection of metabolites (e.g., pregnanetriol).
- In classic salt-wasting 21-hydroxylase deficiency:
- Plasma levels of progesterone and 17-hydroxyprogesterone are markedly elevated.
- Urinary levels of 17-ketosteroids and pregnanetriol are elevated.
- Radioimmunoassay
for 17-hydroxyprogesterone
- Imaging: pelvic US
- A pelvic US study demonstrating the presence of müllerian tissues (e.g. uterus) is confirmatory
- Some investigators have suggested that the finding of abnormally enlarged or “cerebriform”-appearing adrenal glands on neonatal ultrasonography, available before biochemical results, may represent the earliest diagnostic tool for CAH
- Increased incidence of upper tract abnormalities (hydronephrosis, duplication) in CAH
- History and Physical Exam
-
- 11β-hydroxylase deficiency
- Hypertension is a common finding
- Believed to be secondary to increased serum levels of deoxycorticosterone (DOC), which are not elevated in 21-hydroxylase deficiency
- Marked virilization occurs in the severe form of the defect and may be as severe as in those patients with a 21-hydroxylase deficiency.
- In the late-onset form, mild virilization occurs in prepubertal and postpubertal patients.
- Diagnosis and Evaluation
- Labs: increased plasma levels of 11-deoxycortisol and 11-DOC.
- Urinary levels of 17-ketosteroids and 17-hydroxycorticoids are increased.
- Pelvic ultrasound evaluation in females confirming the presence of müllerian tissue would be supportive
- Labs: increased plasma levels of 11-deoxycortisol and 11-DOC.
- Hypertension is a common finding
- 3β-hydroxysteroid dehydrogenase deficiency
- Affects the early steps in steroid biosynthesis in both adrenals and gonads
- Various degrees of incomplete masculinization, resulting from a block in testosterone biosynthesis, and salt-wasting aldosterone insufficiency resulting from impaired synthesis of aldosterone and cortisol.
- The severe form leads to impaired synthesis of aldosterone, cortisol, and sex steroids.
- Males with this deficiency usually exhibit incomplete virilization of the external genitalia, with a small phallus, hypospadias with labioscrotal fusion, a urogenital sinus, and a blind-ending vaginal pouch. Testes are often scrotal, and wolffian duct-derived structures develop normally.
- As with other defects in testosterone biosynthesis, in which normal Sertoli cell function is preserved, müllerian-derived structures structures are absent
- Diagnosis and Evaluation
- Should be considered in 46,XY males with ambiguous genitalia and signs of adrenal insufficiency
- Labs: increased serum levels of 17-hydroxypregnenolone and dehydroepiandrosterone (DHEA)
- Pelvic ultrasound evaluation in females confirming the presence of müllerian tissue would be supportive
- Management
- Glucocorticoids
- “Prophylactic” adrenalectomy
- Considered for selected patients is based on the premise that in certain patients it is more difficult to maintain adrenal suppression than to prevent adrenal crises. Clinically, these patients are the salt wasters and extremely virilized females.
- For the 25% of CAH patients who completely lack 21-hydroxylase enzyme activity and therefore produce neither cortisol nor aldosterone, adrenalectomy may be a practical approach
- Prognosis
- Long-term fertility in males and feminization, menstruation, and fertility in females can be anticipated in the well-treated patient.
- Males with CAH must be followed for testicular adrenal rest tumors as a potential cause of infertility. This is ideally performed with annual screening testicular ultrasonography
- In patients with CAH, adrenal rests along the spermatic cord may become quite large.
- Diagnosing and treating the CAH prenatally
- Diagnosis is made during the first trimester, usually by HLA genotyping or by DNA analysis of genes by chorionic villus sampling at 9-11 weeks’ gestation
- Management
- Treatment of the mother with dexamethasone, which crosses the placenta, suppresses fetal secretion of ACTH, thereby preventing virilization of the genitalia.
- Once pregnancy is confirmed, treatment should be initiated before 9 weeks after the last menstrual period.
- However, a diagnosis of CAH cannot be confirmed before therapy is initiated because the diagnosis is usually made at 9-11 weeks’ gestation. Therefore, if treatment is initiated for all at-risk fetuses, 7/8 may be treated unnecessarily before confirmatory diagnosis.
46,XY Disorder of Sex Development (Under-masculinized Male)
- Refers to 46,XY individuals with differentiated testes who exhibit varying degrees of feminization phenotypically
- Impaired male differentiation in these patients is secondary to:
- Inadequate secretion of testosterone by the testes at the necessary period in development
- Inability of target tissue to respond to androgen appropriately
- Impaired production or action of MIS
- Inadequate secretion of testosterone by the testes at the necessary period in development
- Leydig Cell Aplasia (Luteinizing Hormone Receptor Abnormality)
- Autosomal recessive trait
- Expressed only in males
- Low testosterone level is noted in conjunction with an elevated LH concentration.
- The absence of a rise in serum testosterone level after hCG stimulation is characteristic of this disorder
- There are no müllerian structures, consistent with functioning Sertoli cells, and the vagina is short
- Clinical diagnosis is typically made as a result of sexual infantilism and the absence of development of secondary sexual characteristics or the discovery of palpable gonads in the inguinal canal or labia on physical examination.
- The differential diagnosis includes androgen insensitivity syndrome or a terminal defect in androgen synthesis.
- Disorders of Testosterone Biosynthesis
- A defect in any of the 5 enzymes required for the conversion of cholesterol to testosterone can cause incomplete (or absent) virilization of the male fetus during embryogenesis.
- Autosomal recessive for all 5 enzyme deficiencies
- The first 3 enzymes are present in both adrenals and testes. Therefore, their deficiency results in impaired synthesis of glucocorticoids and mineralocorticoids in addition to testosterone
- StAR (Cholesterol Side Chain Cleavage Enzyme) Deficiency
- The steroidogenic acute regulatory protein (StAR) enzyme is involved in cholesterol side chain cleavage
- Affected 46,XY individuals have female or ambiguous external genitalia with a blind-ending vaginal pouch; intra-abdominal, inguinal, or labial testes; and absence of müllerian structures, consistent with functioning Sertoli cells; Wolffian duct-derived structures are present but rudimentary.
- Infants are often seen in the first few weeks of life with severe adrenal insufficiency and salt wasting, but delayed presentation has been reported
- Diagnosis should be considered in any neonate with nonvirilized female external genitalia and evidence of cortisol and aldosterone deficiency with hyponatremia, hyperkalemia, and metabolic acidosis.
- CT demonstrates large, lipid-laden adrenal glands
- Management: similar to 21-hydroxylase deficiency
- 3β-Hydroxysteroid Dehydrogenase Deficiency (see above)
- 17α-Hydroxylase Deficiency
- 17α-Hydroxylase catalyzes the conversion of pregnenolone and progesterone to 17α- hydroxypregnenolone and 17-hydroxyprogesterone, respectively, in adrenal and gonadal steroidogenesis.
- A deficiency in 17α-hydroxylase activity impairs cortisol production, causing ACTH hypersecretion and resulting in increased levels of DOC, corticosterone, and 18-hydroxycorticosterone in the adrenals. These compounds with mineralocorticoid activity produce excess salt and water retention, hypertension, and hypokalemia
- Phenotype varies from female external genitalia with a blind-ending vaginal pouch to perineal hypospadias and chordee.
- Diagnosis
- Should be considered in a undervirilized male with hypertension
- Laboratory investigations: elevated serum progesterone, DOC, corticosterone, 18-hydroxycorticosterone, and ACTH
- Management
- Glucocorticoid replacement brings blood pressure and hypokalemia back to normal by suppressing ACTH and hence adrenal cortical stimulation
- Reconstruction of the external genitalia
- Appropriate sex steroid replacement at puberty
- 17,20-Lyase Deficiency
- Aldosterone, cortisol, and ACTH secretion are normal.
- Hypertension does not result.
- Impaired biosynthesis of testosterone in the 46,XY individual results typically in ambiguous rather than totally female genitalia at birth.
- The deficient masculinization of the external genitalia can range from severe, resulting in a female gender assignment in the neonate, to mild, resulting only in hypospadias.
- At puberty, the secretion of testicular androgen remains low
- Diagnosis
- May be suspected in undervirilized males with absent müllerian-derived structures and no defect in glucocorticoid or mineralocorticoid synthesis.
- At the time of expected pubertal development, patients may have failure to develop secondary sexual characteristics and elevated gonadotropin levels.
- May be made prepubertally using hCG and ACTH stimulation
- 17β-Hydroxysteroid Oxidoreductase Deficiency
- Last enzyme in the testosterone biosynthetic pathway
- Catalyzes the conversion of androstenedione to testosterone, DHEA to androstanediol, and estrone to estradiol.
- Clinically similar to 5α-reductase deficiency
- At birth, affected individuals appear to have a normal female phenotype, without significant evidence of virilization. Therefore a female gender assignment is usually made. However, these individuals have well-differentiated testes located intraabdominally, inguinally, or in the labia and no müllerian structures.
- In the prepubertal patient, plasma androstenedione and estrone levels may not be increased. At puberty, androstenedione, the immediate precursor of testosterone, is increased to 10-15x the normal plasma concentration. Earlier precursors are within normal levels. Plasma testosterone is in the low-normal range. Serum levels of LH and FSH are markedly elevated, typically 4-6x normal.
- At puberty, there is phallic growth and progressive development of male secondary sexual characteristics. The late onset of virilization is related to the pubertal increase in gonadotropin production, which may partially overcome the block in testosterone biosynthesis.
- The diagnosis is rarely made in the neonatal period. An hCG stimulation test resulting in an increased testosterone-to-androstenedione ratio would confirm the diagnosis and differentiate this condition from androgen insensitivity.
- The primary management issue has been gender assignment.
- Cytochrome P450 Oxidoreductase Deficiency
- Cytochrome P450 oxidoreductase is a cofactor to all microsomal P450 enzymes, including 17-hydroxylase, 17,20-lyase, 21-hydroxylase, and aromatase
- Causes of both 46,XY and 46,XX DSDs.
- A defect in any of the 5 enzymes required for the conversion of cholesterol to testosterone can cause incomplete (or absent) virilization of the male fetus during embryogenesis.
- Inability of target tissue to respond to androgen appropriately
- Disorders of androgen receptor function represent the most common definable cause of 46,XY DSD or the undervirilized male
- These patients characteristically have a 46,XY karyotype and testes and a spectrum of phenotypic abnormalities that vary from
- Complete external feminization: complete androgen insensitivity syndrome
- Ambiguous genitalia: partial androgen insensitivity
- Phenotypically infertile male
- In disorders of the androgen receptor, such as androgen insensitivity syndrome, testosterone production is normal but the hormone is unable to reach the nucleus and interact with DNA.
- Complete (Severe) Androgen Insensitivity Syndrome (CAIS)
- Incidence of 1 in 20,000 to 1 in 60,000 males
- X-linked trait
- The androgen receptor has been mapped to the X chromosome
- Males have only one copy of this gene
- Point mutations of the gene account for > 90% of cases of androgen insensitivity
- Characterized by 46,XY karyotype, bilateral testes, female-appearing external genitalia, and absence of müllerian-derived structures, consistent with functioning Sertoli cells
- Patients have a normal female phenotype with the exception of diminished axillary and pubic hair.
- Breast development and body habitus are feminine in character
- At puberty, gonadotropin levels rise, leading to increased levels of plasma estradiol, which results in feminization, including breast development.
- ≈80% will have "normal" appearing, although the vagina is short and blind ending.
- Breast development and body habitus are feminine in character
- Wolffian-derived structures may be present
- Screening of the paratesticular area revealed well-developed epididymis and/or vasa deferentia in 42% of patients
- The testes may be found in the labia, inguinal canal, or abdomen
- Patients have a normal female phenotype with the exception of diminished axillary and pubic hair.
- Rarely diagnosed in the neonatal period; patients typically present by one of 5 different means:
- Fetal karyotype (46 XY) incongruent with newborn infant's phenotype (5% of patients)
- Relative or family member with CAIS, with patient diagnosed due to recommendation for genetic screening (15% of patients)
- Ambiguous genitalia at birth, i.e., female phenotype with palpable gonads or mild to moderate clitorimegaly (20% of patients)
- Primary amenorrhea (30% of patients)
- Testicle found within a inguinal hernia at the time of surgical repair (30% of patients)
- Vaginoscopy to confirm the presence of a cervix or endoscopy through a hernia sac to identify an intra-abdominal testis at the time of inguinal herniorrhaphy in female patients is a prudent maneuver.
- Diagnosis and Evaluation
- History and Physical Exam
- May readily be made in the postpubertal patient on the basis of clinical and hormonal findings of amenorrhea, absence of pubic hair, or inguinal hernias containing testes
- Vaginal examination confirms a blind-ending vagina without a cervix
- Labs
- Karyotype: 46,XY
- Endocrine evaluation
- Neonatal period: normal male levels of testosterone, DHT, and gonadotropins
- Puberty:
- Testosterone production and secretion by the Leydig cells is normal
- LH is increased because of the apparent lack of testosterone by the target organs including the pituitary gland that not recognize the testosterone due to faulty receptors.
- FSH is normal since it is controlled by inhibin
- In the prepubertal child, diagnosis is more difficult and requires an hCG stimulation test.
- [Unlike 17β-Hydroxysteroid Oxidoreductase Deficiency, hCG stimulation test in androgen insensitivity would not result in an increased testosterone-to-androstenedione ratio]
- PCR can be used characterize the androgen receptor gene
- Imaging
- Pelvic US confirms the absence of müllerian-derived structures
- History and Physical Exam
- Management
- Relates primarily to the optimal timing of gonadectomy
- Patients with CAIS will have a substantial increased risk of developing a testicular seminoma
- If the testis is left in situ, ≈20% of the patients will have developed a testicular malignancy by the age of 30.
- Removal of the testicles are, therefore, strongly recommended either prior to or immediately following pubescence
- Because the testes produce estradiol, which results in the appropriate changes for the female phenotype, it is considered by many preferable to leave the testes in situ until puberty is complete. In general, delayed gonadectomy after puberty is believed to be safe
- Gonadoblastoma is a tumor that is associated with disorders of sex development. Specifically, it is found in infants noted to have partial or pure gonadal dysgenesis (46 XY or 46 XY/XO genotypes) and is not associated with complete androgen insensitivity syndrome
- After orchiectomy, cyclic estrogen-progestin therapy is begun.
- Patients with CAIS will have a substantial increased risk of developing a testicular seminoma
- All studies CAIS have been in patients with an unequivocal female gender identity, consistent with androgen resistance of brain tissue as well.
- Relates primarily to the optimal timing of gonadectomy
- Syndrome of Partial Androgen Insensitivity Syndrome (PAIS)
- Also known as Reifenstein syndrome
- X-linked disorder
- A disorder of androgen receptor quantity or function
- Ambiguious external genitalia to varying degrees, from hypospadias and a pseudovagina to gynecomastia and azoospermia
- Classic phenotype is that of a male with perineoscrotal hypospadias, cryptorchidism, rudimentary wolffian-derived structures, gynecomastia, and infertility.
- Diagnosis
- Can be difficult; a family history consistent with X-linked inheritance of ambiguous genitalia is suggestive of the disorder
- In the neonatal period, it may be made in the setting of a 46,XY karyotype, ambiguous external genitalia, and absent müllerian-mullerian structures on pelvic ultrasound.
- Endocrine evaluation demonstrates normal male levels of testosterone and gonadotropins, and a normal testosterone/DHT ratio.
- An hCG stimulation test and characterization of the androgen receptor gene in serum DNA by PCR should confirm the diagnosis.
- Management
- Must be individualized depending on the degree of genital ambiguity
- A course of androgen injections in early infancy is often used to assess androgen responsiveness, which can aid in gender assignment.
- Mild Androgen Insensitivity Syndrome
- Variety of mutations, usually quite discrete within the androgen receptor gene, that accounts for the infertility in these patients
- This suggests that infertility in otherwise normal males may be the clinical manifestation of mild androgen insensitivity, representing the far end of a variable phenotypic spectrum.
- May have normal phenotypically or have a history of mild hypospadias repair but are azoospermic or severely oligospermic.
- Endocrine evaluation demonstrates normal to elevated serum testosterone levels with normal to elevated LH levels.
- Variety of mutations, usually quite discrete within the androgen receptor gene, that accounts for the infertility in these patients
- 5α-Reductase Deficiency
- Autosomal recessive, only homozygous males are affected
- The type 2 isoenzyme is affected in patients with 5α-reductase deficiency, resulting in an increased testosterone/DHT ratio owing to a reduced testosterone-to-DHT conversion rate
- Phenotype that may vary from normal female to markedly ambiguous genitalia (more common) to penoscrotal hypospadias to the rare, isolated microphallus
- Typically the phallus is quite small, appearing as a normal or enlarged clitoris
- Presence of labioscrotal fusion; vaginal pouch is short and blind ending.
- Testes and epididymides are located in the labia, inguinal canals, or abdomen; and the vasa terminate in the blind-ending vaginal pouch.
- At puberty, partial masculinization occurs with an increase in muscle mass, development of male body habitus, increase in phallic size, and onset of erections, virilization is presumed to occur because the androgen receptor binds markedly higher levels of testosterone at low affinity or because of the normal increase at puberty in the activity of the 5α-reductase type 1 isoform, resulting in sufficient DHT for virilization
- Other secondary sexual characteristics, including enlargement of the prostate and hairline recession, do not develop.
- Although DHT appears to be critical for the development of normal external genitalia in utero, testosterone alone appears sufficient for wolffian duct development.
- Endocrine evaluaiton: elevated mean plasma testosterone but low DHT levels. After hCG stimulation, the testosterone-to-DHT ratio increases to > 20:1.
- Strong tendency toward reversal of gender identity in 5α-reductase deficiency
- Impaired production or action of MIS
- Persistent Müllerian Duct Syndrome
- Patients with a 46,XY karyotype and normal male external genitalia but internal müllerian duct structures
- DSD resulting from defective AMH signaling
- Aberrant MIS function may be secondary to defects in the gene for MIS or in the gene for the MIS receptor
- Genetically heterogeneous disorder in which some subjects have a defect in the gene for MIS and others have a defect in the gene for its type II receptor. The condition may occur sporadically or may be inherited as an X-linked (or autosomal dominant, sex-limited) trait
- Aberrant MIS function may be secondary to defects in the gene for MIS or in the gene for the MIS receptor
- Typically, these phenotypic males have unilateral or bilateral undescended testes, bilateral fallopian tubes, a uterus, and an upper vagina draining into a prostatic utricle.
- Suggested by the presence of Mullerian structures (uterus, fallopian tube) attached to an undescended testicle (more commonly intra-abdominal)
- Usually an intraoperative finding
- Patients should have AMH levels checked and be referred to endocrinology/genetics for investigation
- Rarely, can lead to both testicles occupying the same side of the abdomen (transverse testicular ectopia)
- Management
- All patients are phenotypic males who require orchidopexy
- The cases of adult patients with associated testis tumor (most commonly seminoma) probably reflect the increased risk of malignancy in intra-abdominal undescended testes.
- Malignancies have been reported in retained müllerian remnants and at times their attachments can hinder the performance of a tension-free orchidopexy.
- When Mullerian remnants are found incidentally during an inguinal orchidopexy, the proximal aspect of the fallopian tube can be transected and removed with the uterus, leaving its distal component attached to the vas deferens, allowing the testis to be brought to a scrotal position Such a maneuver avoids separation of the tube from the cord structures, protecting the deferential and testicular blood supply.
- Careful surgical excision is required as the vasa deferentia are in close proximity to the uterus and proximal vagina
- When Mullerian remnants are found incidentally during an inguinal orchidopexy, the proximal aspect of the fallopian tube can be transected and removed with the uterus, leaving its distal component attached to the vas deferens, allowing the testis to be brought to a scrotal position Such a maneuver avoids separation of the tube from the cord structures, protecting the deferential and testicular blood supply.
- Persistent Müllerian Duct Syndrome
- Leydig Cell Aplasia (Luteinizing Hormone Receptor Abnormality)
Unclassified Forms
- Embryonic Testicular Regression and Bilateral Vanishing Testes Syndromes
- Karyotype: 46XY
- Two seperate syndromes characterized by bilateral absent testes in whom there is clear evidence of testicular function at some point during embryogenesis
- Distinguished from 46,XY pure gonadal dysgenesis, in which there is no evidence of testicular function in utero
- Embryonic testicular regression refers to loss of testicular tissue within the first trimester and is associated with ambiguity of external genitalia
- Bilateral vanishing testes syndrome refers to individuals in whom male sexual differentiation of ducts and genitalia took place but regression of testicular tissue occurred subsequently in utero.
- Regression of the testes in utero is caused by a genetic mutation, a teratogen, or bilateral torsion
- Clinically, these two syndromes represent a spectrum of phenotypes ranging in severity from complete female, to varying degrees of genital ambiguity in the embryonic testicular regression syndrome, to a normal male phenotype with microphallus and empty scrotum in the bilateral vanishing testes syndrome.
- The diagnosis can be made on the basis of a 46,XY karyotype, castrate levels of testosterone, elevated serum LH and FSH, and undetectable MIS level
- Management
- Dictated by clinical spectrum of either disorder
- Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome
- Characterized by 46,XX karyotype, and are normal-appearing female phenotype with normal secondary sex characteristics, and congenital absence of the uterus and vagina.
- The external genitalia appear normal, but only a shallow vaginal pouch is present.
- In the typical form of the syndrome there is symmetrical anatomy with absence of both vagina and uterus.
- Normal ovaries and fallopian tubes are present, and ovarian function is normal
- Most common clinical presentation is primary amenorrhea, but patients may have infertility or dyspareunia
- Upper urinary tract anomalies occur in approximately 1/3 of patients and include renal agenesis, pelvic kidney, and horseshoe kidney
- Urinary tract anomalies occur more commonly in patients with the atypical form
- Imaging with ultrasonography and MRI may define müllerian anatomy accurately and distinguish between typical and atypical forms of the disorder
- Managment entails creation of a neovagina, by means of dilation or surgically, to allow for sexual function
- Characterized by 46,XX karyotype, and are normal-appearing female phenotype with normal secondary sex characteristics, and congenital absence of the uterus and vagina.
Approach to the newborn with ambiguous genitalia
- Team’s goal should be to make a precise diagnosis of the disorder (which can be achieved in most cases) and, with the involvement of the parents, to assign a proper sex of rearing based on the diagnosis, the status of the child’s anatomy, and the functional potential of the genitalia and reproductive tract.
- Diagnosis and Evaluation
- History and physical exam (including stretched penile length, palpable testis, hypospadias, etc.)
- Laboratory investigations (4):
- Karyotype
- Serum electrolytes (rule out salt-wasting from CAH)
- Testosterone, DHT
- 17-hydroxyprogesterone (rule out 21-OH deficiency; should not be measured until day 3-4)
- Imaging
- Pelvis US to determine presence of Mullerian-derived structures
- History and Physical exam
- History
- Family history of
- Infant death within the family might suggest the possibility of CAH
- Infertility, amenorrhea, or hirsutism might also suggest possible familial patterns of intersex states
- Maternal use of medications, in particular steroids or contraceptives, during the pregnancy
- Family history of
- Physical exam
- The critical component is the presence of one or two palpable gonads. This finding effectively rules out over-masculinization [i.e. CAH] of the female.
- Because ovaries do not descend, a distinctly palpable gonad along the pathway of descent is highly suggestive of a testis.
- The patient with bilateral impalpable testes or a unilateral impalpable testis and hypospadias should be regarded as having a DSD until proven otherwise, whether or not the genitalia appear ambiguous
- Incidence of DSD with
- Unilateral undescended testis: 30%
- Unilateral undescened palpable: 15%
- Unilateral undescened impalpable: 50%
- Unilateral undescened impalpable with posterior urethral meatus: 65%
- Unilateral undescened impalpable with anterior urethral meatus: 5-8%
- Bilateral undescended testes and hypospadias: 32%
- Bilateral undescended palpable: 16%
- Bilateral undescended impalpable: 50%
- Unilateral undescended testis: 30%
- Incidence of DSD with
- Penile size should be assessed and an accurate measure of stretched penile length recorded.
- Recall, the mean stretched penile length in full-term males born in the United States is 3.5 cm
- Presence of a uterus can be assessed by physical exam but a more precise means of assessing müllerian anatomy is by pelvic US, which may be performed immediately in the neonatal period.
- In addition to defining müllerian anatomy and confirming the presence or absence of a uterus, the gonads and adrenals should be studied.
- The critical component is the presence of one or two palpable gonads. This finding effectively rules out over-masculinization [i.e. CAH] of the female.
- History
- Labs
- Karyotype
- Should be obtained within the immediate neonatal period
- Serum electrolytes
- Should be sent immediately sent to rule out a saltwasting form of CAH
- Testosterone and DHT
- Should be measured early
- 17-hydroxyprogesterone
- Should not be measured until day 3 or 4 to rule out 21-hydroxylase deficiency, because the stress of delivery may result in physiologic elevation of this steroid precursor in the first 1 or 2 days of life.
- hCG stimulation test
- In the absence of palpable testes, the presence or absence of testicular tissue should be determined by documentation of a markedly elevated LH level, consistent with anorchia, or by means of an hCG stimulation test, which can demonstrate normally functioning testicular tissue.
- In addition to ruling out anorchia, the [hCG stimulation] study can enable diagnosis of 5α-reductase deficiency (by virtue of an increased ratio of testosterone to DHT) and can help distinguish between impaired testosterone synthesis (deficient response to hCG) and androgen insensitivity (normal response to hCG).
- In the absence of palpable testes, the presence or absence of testicular tissue should be determined by documentation of a markedly elevated LH level, consistent with anorchia, or by means of an hCG stimulation test, which can demonstrate normally functioning testicular tissue.
- Serum MIS
- Should be included as a marker of the presence of testicular tissue
- PCR characterization of the androgen receptor in venous blood DNA
- May define the precise genetic abnormality responsible for a given DSD, be it abnormal androgen receptor or an enzyme abnormality.
- Karyotype
- Imaging
- Pelvis US to determine presence of Mullerian-derived structures
- Laparotomy or laparoscopy in this setting remains a diagnostic maneuver; removal of gonads or reproductive organs should be deferred until the final pathology report is available and a gender has been assigned.
- Management
- Gender assignment
- Issues related to the diagnosis-specific potential for normal sexual functioning and fertility and the risk of gonadal malignancy should be addressed.
- In the setting of a 46,XX karyotype and masculinized female, gender assignment is usually appropriately female.
- In CAH, cortisol suppresses the undesired androgen; and if maternal androgen is responsible for virilization, its discontinued stimulation is corrective. In both cases there are normal ovaries and müllerian-derived structure, and a normal reproductive potential exists.
- If the karyotype is 46,XY, the issue is a more complex one and includes factors such as penile length and evidence of androgen insensitivity.
- Gender role refers to aspects of behavior that distinguish males and females. The development of gender identity is poorly understood, but is influenced by prenatal and postnatal factors. Individual conflicts with gender identity are central to the concept of gender dysphoria
- The best predictor of adult gender identity is initial gender assignment
- Deferring the issue of gender assignment until patients reach an age at which they may declare their own gender identity is not recommended
- Parameters of optimal gender policy outlined in the management of ambiguous genitalia by:
- Reproductive potential (if attainable at all)
- Good sexual function
- Minimal medical procedures
- An overall gender-appropriate appearance
- A stable gender identity
- Psychosocial well-being
- Gender assignment
References
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, vol 4, chap 150