Male Reproductive Physiology: Difference between revisions
Jump to navigation
Jump to search
Urology4all (talk | contribs) |
Urology4all (talk | contribs) |
||
| Line 286: | Line 286: | ||
**'''An age-related decrease in sperm production in older testes appears to stem from decreased germ cell proliferation, rather than increased cellular degeneration''' | **'''An age-related decrease in sperm production in older testes appears to stem from decreased germ cell proliferation, rather than increased cellular degeneration''' | ||
**'''Increasing paternal age increases the fraction of sperm with sex chromosomal aneuploidies.''' '''However, little evidence to support a paternal age-related increase in aneuploid births,''' except possibly trisomy 21 and disomy 1 | **'''Increasing paternal age increases the fraction of sperm with sex chromosomal aneuploidies.''' '''However, little evidence to support a paternal age-related increase in aneuploid births,''' except possibly trisomy 21 and disomy 1 | ||
== Next Chapter: [[Epidemiology and Etiology|Epidemiology and Etiology of Infertility]]== | |||
== References== | == References== | ||
Revision as of 09:55, 2 March 2024
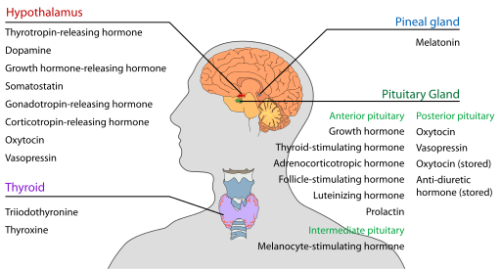
Reproductive (Hypothalamus, Pituitary, Testis) Axis
Hypothalamus
- Produces GnRH
- GnRH
- Simulates production and release of LH and FSH from anterior pituitary
- Patterns of secretion (3):
- Seasonal (peaking in spring)
- Circadian (highest testosterone in morning)
- Pulsatile, peaks every 90-120 minutes
- Pulsatility arises at puberty, around age 12
- GnRH

Anterior Pituitary
- Produces (6):
- Luteinizing hormone
- Follicle-stimulating hormone
- Growth hormone
- Thyroid-stimulating hormone
- Adrenocorticotropic hormone
- Prolactin
- LH and FSH act only on gonads
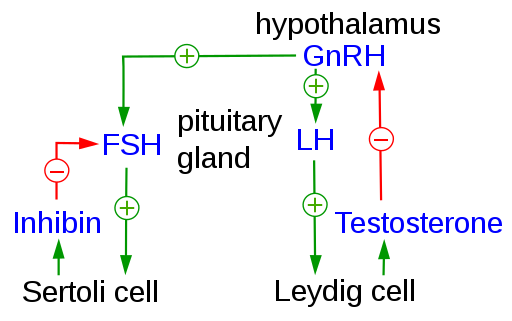
- Luteinizing hormone (LH)
- Function:
- Stimulates steroidogenesis within Leydig cells
- Most important regulator of testosterone production
- Clinical implication: infertilty secondary to reduced LH can be treated with hCG hormonal therapy, which is an analogue of LH
- Most important regulator of testosterone production
- Stimulates steroidogenesis within Leydig cells
- Secretion
- Regulated by negative feedback from estrogens (primary mechanism) and androgens
- Estradiol feedback occurs mainly at pituitary but also negative-feedback on hypothalamus
- Testosterone feedback occurs mainly at hypothalamus
- Clinical implication: exogenous testosterone inhibits production of FSH and LH and is associated with decreased serum FSH and LH
- Regulated by negative feedback from estrogens (primary mechanism) and androgens
- Function:
- Follicle-stimulating hormone (FSH)
- Function:
- Stimulates Sertoli cells
- Major stimulator of seminiferous tubule growth
- Essential for initiation of spermatogensis at puberty but not essential for spermatogenesis in acquired infertility (i.e. after puberty)
- Clinical implication: infertilty can be treated with hCG hormonal therapy monotherapy without concomitant recombinant FSH
- Stimulates Sertoli cells
- Secretion
- Stimulated by (2):
- Estrogen
- Activin
- Inhibited by (1):
- Inhibin
- Thought to account for the relative secretory independence of FSH from GnRH secretion
- Stimulated by (2):
- Function:
- Activin
- Secreted by Leydig cells
- Inhibin-B
- Secreted by Sertoli cells
- Production is stimulated by FSH
- Acts by negative feedback at the pituitary [Campbell’s 12th edition, page 1319 says both “hypothalamus or pituitary”, Wikipedia says “Inhibin does not inhibit the secretion of GnRH from the hypothalamus", 2019 AUA Update on Exogenous Testosterone and Male Reproduction agrees with Wikipedia] to inhibit FSH production
- Inhibin and activin can influence steroid production by Leydig cells
- Prolactin
- Normal levels may increase concentration of LH receptors on Leydig cells and sustain normal, high intratesticular testosterone levels
- May also potentiate the effects of androgens on growth and secretions of male accessory sex glands
- Hyperprolactinemia abolishes GnRH pulsatility and is a cause of infertility
Testis
- See Testis Anatomy Notes
- After binding LH, Leydig cells transport cholesterol into mitochondria and produce testosterone.
Development
- SRY gene on Y chromosome critical for sex determination
- Once sex determined, Leydig cells make:
- Testosterone
- Stimulates development of INTERNAL genitalia except prostate (seminal vesicles, epididymis, vas deferens, ejaculatory ducts)
- DHT
- Stimulates development of EXTERNAL genitalia (penis, scrotum) and prostate
- Insulin-like growth factor-3
- Stimulates transabdominal testis migration into the scrotum
- Activin (see above)
- Testosterone
- Sertoli cells synthesize
- Inhibin (see above)
- Mullerian-inhibiting substance (MIS), which prevents the Mullerian duct from developing into uterus and fallopian tubes
- Androgen-binding protein
- Binds testosterone and maintains high levels of androgen within the seminiferous tubules
- Testosterone peaks at 3 stages of life:
- 13 weeks
- ≈2 months of age
- Puberty
Testosterone
- Most important circulating androgen
- Production
- 90% produced by the testes and 10% by the adrenals
- Normal testosterone production is 5 g/day
- Circulation
- Circulates in bound (98%) and free (2%) form
- Binding proteins (3):
- Albumin (50%, loosely-bound)
- Sex hormone-binding globulin ([SHBG], 44%, tightly-bound)
- Produced in the liver
- Corticotropin-binding globulin (4%, loosely-bound)
- The free and loosely-bound (albumin, corticortropin-binding) testosterone fractions combined are known as bioavailable testosterone
- Only bioavailable testosterone can enter cells and have an effect on the androgen receptor.
- Free and total testosterone measurements are most accurate when done by equilibrium dialysis but in the absence of this assay, they can be calculated from total testosterone, SHBG and albumin.
- Serum testosterone levels <300 ng/dL considered low
- Calculated free testosterone <6.5 ng/dL considered low
- Intratesticular testosterone levels are 50-100x those of the circulating serum levels and are necessary for appropriate spermatogenic function.
- SHBG
- Conditions associated with decreased SHBG (so expect higher proportion of bioavailable testosterone for same total testosterone level)
- Obesity
- Nephrotic syndrome
- Hypothyroidism
- Use of glucocorticoids, progestins, and androgenic steroids
- Acromegaly
- Diabetes mellitus
- Conditions associated with increased SHBG [so expect lower proportion of bioavailable testosterone for same total testosterone level]
- Aging
- Hepatitis and cirrhosis/liver failure
- Hyperthyroidism
- Use of anticonvulsants
- Use of estrogens
- HIV
- Conditions associated with decreased SHBG (so expect higher proportion of bioavailable testosterone for same total testosterone level)
- Metabolism
- Occurs primary in the liver
- Metabolized by
- Aromatase into estradiol
- Estradiol (E2)
- Inhibits LH secretion
- Most potent regulator of the HPG axis in the male
- Promotes bone health and libido
- Inhibits LH secretion
- Estradiol (E2)
- 5α-reductase to dihydrotestosterone (DHT), mainly in the target organs
- Following passive diffusion through the cell membrane into the cytoplasm, testosterone undergoes conversion to dihydrotestosterone (DHT) through the action of the enzyme 5α-reductase
- Both testosterone and DHT exert their biologic effects by binding to the AR in the cytoplasm, promoting the association of AR coregulators. The complex then translocates to the nucleus and binds to androgen response elements in the promoter regions of target genes
- The relative potency of testosterone and DHT are similar (as defined by the ability to cause half-maximal response in a prostate regrowth model), however, if the conversion of testosterone to DHT is blocked by the 5α-reductase inhibitor finasteride, 13-fold more testosterone is required for the same effect.
- DHT is in high concentrations in the prostate and hair follicles
- Isoforms of 5α-reductase (2):
- Type 1: localized in the non-genital skin, liver, brain, prostate, and testis
- Inhibited by finasteride and dusteride
- Type 2: active in the classical androgen-dependent tissues (epididymis, genitalia, seminal vesicle, testis, and prostate) but also in liver, uterus, breast, hair follicles, and placenta
- Inhibited by dutasteride
- Type 1: localized in the non-genital skin, liver, brain, prostate, and testis
- Aromatase into estradiol
- Half-life of testosterone in plasma is 12 minutes
- Testosterone and DHT contribute to muscle, bone, skin, sperm, brain, nerve development and hematopoiesis.
- Adrenal androgens
- Androstenedione and dehydroepiandrosterone (DHEA)
- Androstenedione is more potent than DHEA
- Production stimulated by ACTH released by the pituitary gland in response to corticotropin-releasing facto; like cortisol, adrenal androgen secretion exhibits circadian patterns.
- Almost entirely bound to albumin
- Relatively weak compared to testosterone and DHT
- Remain normal in men who have undergone orchiectomy and are insufficient to maintain prostatic epithelium in such men.
- Androstenedione and dehydroepiandrosterone (DHEA)
Cardiovascular disease and testosterone
- Androgen deprivation therapy increases cardiovascular risk by affecting various risk factors: increased body weight, decreased insulin sensitivity, altered lipid profile, and increased fat mass
- Coronary artery disease
- The degree of testosterone deficiency has been reported as having an inverse relationship to the severity of CAD
- Exogenous testerone therapy in men with testosterone deficiency improves myocardial ischemia, exercise capacity, and CV risk factors.
- Current guidelines do not recommend offering testosterone deficiency screening to patients with heart disease, nor do they recommend supplementing testosterone therapy to improve outcome
- Cerebral vascular disease
- Testosterone deficiency has also been implicated in the development of cerebral vascular disease
- Proposed mechanisms of testosterone’s action on the cardiovascular system (3):
- Protective effect on endothelial function
- Anti-anginal, anti-inflammatory, anti-ischemia effects
- Testosterone levels correlate negatively with fibrinogen
- TEAAM (Testosterone's Effects on Atherosclerosis Progression in Aging Men) trial
- Population: 156 men age ≥ 60 years or older with low or low-normal testosterone levels
- Randomized to 7.5g of 1% testosterone gel packets daily vs. placebo for 3 years
- Coprimary outcomes:
- Common carotid artery intima-media thickness
- Coronary artery calcium
- Secondary outcomes: sexual function and health-related quality of life
- Results:
- No significant difference in common carotid artery intima-media thickness, coronary artery calcium, sexual desire, erectile function, overall sexual function scores, partner intimacy, and health-related quality of life.
- Hematocrit and prostate-specific antigen levels increased more in testosterone group.
- Effects of Testosterone Administration for 3 Years on Subclinical Atherosclerosis Progression in Older Men With Low or Low-Normal Testosterone Levels: A Randomized Clinical Trial. JAMA. 2015 Aug 11;314(6):570-81. doi: 10.1001/jama.2015.8881.
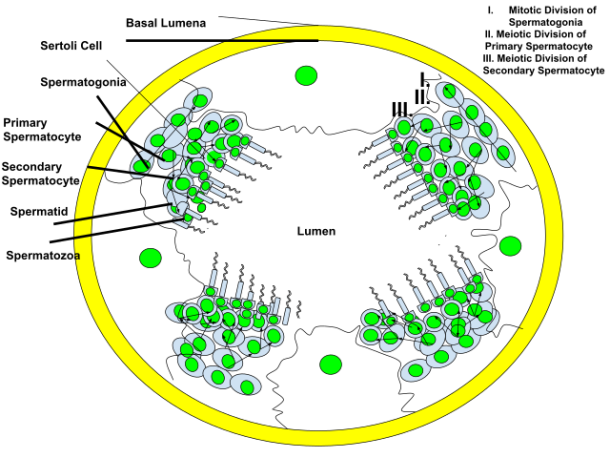
Spermatogenesis
- 13 recognizable germ cell types:
- Dark type A spermatogonia (Ad)
- Pale type A spermatogonia (Ap)
- Type B spermatogonia (B)
- Preleptotene primary spermatocytes (R)
- Leptotene primary spermatocytes (L)
- Zygotene primary spermatocytes (Z)
- Pachytene primary spermatocytes (P)
- Secondary spermatocytes (II)
- Sa, Sb, Sc, Sd1, Sd2 spermatids
- Type A spermatogonia are the only true stem cell in the testis because they can either self-renew or differentiate to become sperm
- Total time to produce an ejaculated sperm ranges from 42 to 76 days, the majority of which is spent in the testicle
- Sperm spend 45 to 60 days developing in the testis and 2 to 12 days in the epididymis. They are not routinely found in the seminal vesicle, and spend only seconds in the ejaculatory ducts and urethra during ejaculation.
- Phases of spermatogenesis (3):
- Proliferative
- Spermatogonia divide to replace their number, or differentiate into daughter cells that become mature gametes
- Meiotic
- Crossing over of sister chromatids, exchange of genetic material, creation of unique daughter cells, and halving the number of chromosomes.
- Haploid gametes differ genetically from their adult precursors.
- Only occurs in gametes
- Mature primary spermatocytes are the first germ cells to undergo meiosis
- Faulty recombination can cause azoospermia and infertility
- The final product of meiosis is the spermatid
- Somatic cells replicate by mitosis, producing genetically identical daughter cells
- Mature primary spermatocytes are the first germ cells to undergo meiosis
- Only occurs in gametes
- Haploid gametes differ genetically from their adult precursors.
- Crossing over of sister chromatids, exchange of genetic material, creation of unique daughter cells, and halving the number of chromosomes.
- Spermiogenesis:
- Cellular remodeling and nuclear compaction of spermatid DNA; spermatids mature to become spermatozoa
- Proliferative
- SpermatOGONIA (least differentiated) --› spermatOCYTE --› spermatID (most differentiated) --› spermatOZOA
Epididymis
- See Epididymis Anatomy Chapter Notes
- Functions (3):
- Sperm transport
- The principal mechanism responsible for moving spermatozoa through the epididymis is probably the spontaneous rhythmic contractions of the contractile cells surrounding the epididymal duct
- Sperm storage
- After migration through the caput (head) and corpus (body) of the epididymis, spermatozoa are retained in the cauda (tail) epididymis for varying lengths of time, depending on the degree of sexual activity
- Maturation of spermatozoa
- Maturation changes (4):
- Improving cell membrane structural integrity
- Increasing fertilization ability
- Improving motility
- Increased capacity for glycolysis
- Sperm fertility maturation in humans is, in most part, achieved at the level of the distal corpus (body) or proximal cauda (tail) epididymis
- Clinical implication: in men with congenital absence of the vas deferens or epididymal obstruction from vasectomy, sperm retreival should target the cauda (tail) for better sperm motlity compared to the caput (head)
- Maturation changes (4):
- Function influenced by temperature
- Sperm transport
- Fate of unejaculated sperm unknown
- Testosterone and DHT are in high concentration
Vas Deferens
- See Vas Deferens Anatomy Chapter Notes
- Functions
- Has both absorptive and secretory functions
- Actively converts testosterone to DHT
- Does not store sperm
- Immediately before emission, with sympathetic stimulation, sperm is rapidly transported from the distal epididymis through the vas deferens to the ejaculatory duct
- After ejaculation, the contents of the vas deferens are propelled back into the epididymis
Seminal vesicles
- Secrete 70-80% of ejaculatory fluid, with the remainder coming from the prostate, vas deferens, and periurethral Cowper glands
- Seminal fluid
- Contains fructose
- Functions:
- Coagulates semen
- Promotes sperm motility
- Increases stability of sperm chromatin
- Suppresses immune activity in the female reproductive tract
- Provides anti-oxidant protection to sperm
- Alkaline pH
- Mixing of alkaline seminal vesicle with acidic prostatic secretions results in human semen having a mildly alkaline pH
- Clinical implication: acidic ejaculate (pH<7.2) is associated with blockage or absence of seminal vesicles
- Mixing of alkaline seminal vesicle with acidic prostatic secretions results in human semen having a mildly alkaline pH
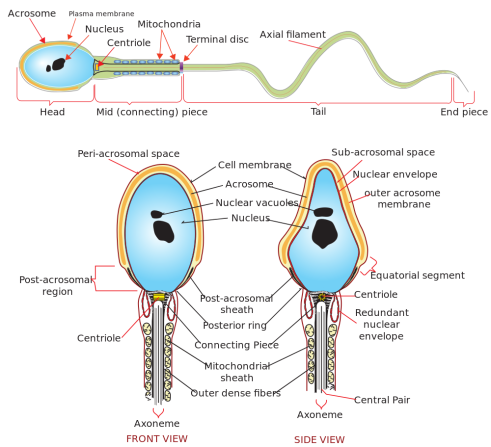
Spermatozoa
- Morphologic sections (3):
- Head (contains a nucleus and an acrosome)
- Acrosome is needed for fertilization (specifically, capacitation)
- Lack of acrosome is associated with round-headed sperm
- Clinical implication: round-headed sperm morphology is associated with infertility
- Neck (connecting piece and proximal centriole)
- Tail (midpiece, principal piece, endpiece)
- The Midpiece in the tail contains the Mitochondria
- Head (contains a nucleus and an acrosome)
- Ciliated cells with a 9+2 axonemal structure that allows motility
- Utilize glucose and fructose (from seminal vesicles) for energy
- Processes that must occur for a sperm to normally fertilize an egg include (4):
- Development of motility
- Acrosome reaction
- Capacitation
- Zona pellucida binding
Aging
- Testosterone decrease with age, causes are multifactorial:
- Fewer Leydig cells
- More testosterone-binding proteins, including SHBG, resulting in decreased bioavailable testosterone
- Loss of diurnal variation of testosterone secretion
- Blunted HPG feedback response to low testosterone (despite generally high levels of gonadotropins) and to GnRH stimulation
- Irregular GnRH pulses that are less effective in stimulating gonadotropin release
- FSH levels increase with age
- Sperm production decrease with age
- An age-related decrease in sperm production in older testes appears to stem from decreased germ cell proliferation, rather than increased cellular degeneration
- Increasing paternal age increases the fraction of sperm with sex chromosomal aneuploidies. However, little evidence to support a paternal age-related increase in aneuploid births, except possibly trisomy 21 and disomy 1
Next Chapter: Epidemiology and Etiology of Infertility
References
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, chap 22
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, chap 23
- Rambhatla A, Mills JN. 2017 AUA Update on Primary, Secondary and Adult Onset Hypogonadism: Diagnosis and Treatment