Infertility: Epidemiology and Etiology
Definitions
- Definition of infertility: a disease of the reproductive system defined by the failure to achieve a clinical pregnancy after ≥12 months of regular unprotected sexual intercourse★
- For those who ultimately become pregnant, cumulative pregnancy rate at:[1]
- 6 months: ≈75%
- 12 months: ≈85%
- 24 months: >90%
- For those who ultimately become pregnant, cumulative pregnancy rate at:[1]
- Idiopathic vs. unexplained infertility§
- Idiopathic infertility: unable to identify etiology of the abnormal semen analysis
- Unexplained infertility: normal semen analysis and normal partner evaluation with unclear reason for infertility
- In some cases, patients with normal semen analyses have sperm that do not function in a manner necessary for fertility
Classification
- Primary vs. secondary
- Primary male infertility: male who has never initiated a clinical pregnancy and meets the criteria of being classified as infertile
- Secondary male infertility: couple where the man is unable to initiate a clinical pregnancy, but who had previously initiated a clinical pregnancy (with the same or different sexual partner)
Epidemiology of Infertility
- Prevalence
- Varies depending on definition of infertility, source of data, and population
- Prevalence in Canada: ≈11%
- Data from the Infertility component in the 2009–2010 Canadian Community Health Survey
- Definition of infertility: did not become pregnant after exposure to the risk of conception during the previous 12 months.
- Risk of conception: did not use any form of birth control within the past 12 months, reported having sexual intercourse in the past 12 months, and reported ever having tried to become pregnant with their current partner.
- Bushnik, Tracey, et al. "Estimating the prevalence of infertility in Canada." Human reproduction 27.3 (2012): 738-746.
- Semen parameters peak after 1 or 2 days of abstinence, then decline.
- To increase the probability of pregnancy, intercourse every day around the time of ovulation is optimal
- To assess bulk seminal parameters, a single day of abstinence is optimal
Risk Factors for Infertility
- Age of the female partner is the single most important factor when predicting the chances of conception for a couple.
- Female fecundity declines precipitously after age 35
- Fertility decreases by almost 50% in women in their late 30’s compared to women in their 20’s.
- In women under 35 years of age, infertility is considered present after 12 months of attempting to conceive. This duration is shortened in women over the age of 35 years to 6 months.
- Female fecundity declines precipitously after age 35
- Male factors[2]
- Solely responsible in ≈20% of all infertile couples
- Contributes to 30-40% of all infertile couples
- ≈30% female factor solely responsible, ≈10% unexplained
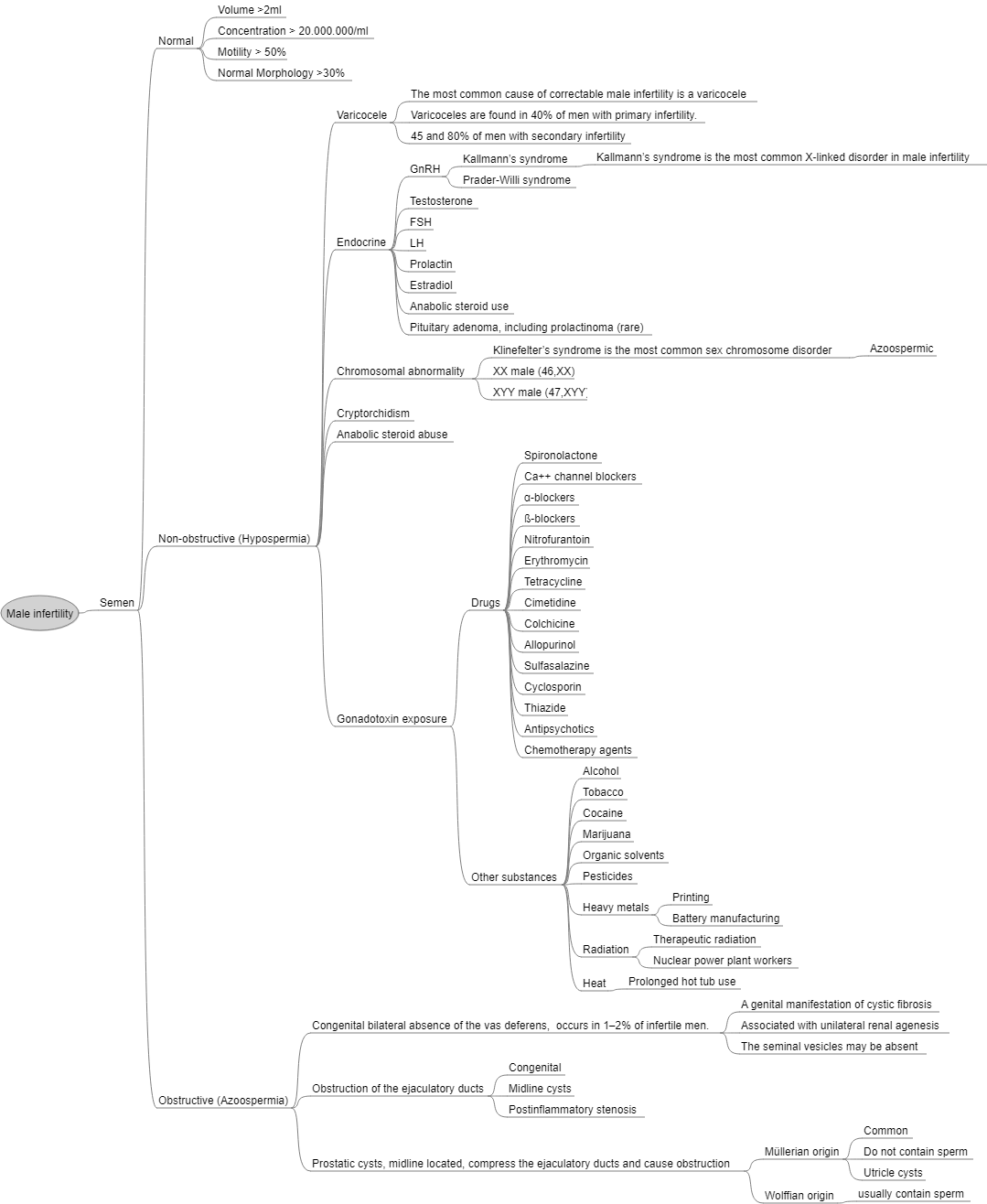
Risk Factors for Male Infertility
Most common causes
- Idiopathic (33%)
- Varicocele (27%)
- Obstruction (15%)
- Endocrinopathy (10%), hypogonadism being most common
Classified (3):
- Pre-testicular
- Testicular
- Post-testicular
Pre-testicular (hypogonadotropic hypogonadism)
Congenital
- Kallmann syndrome
- X-linked recessive disorder
- Characterized by decreased pituitary hormone secretion
- Associated with anosmia
- Presence can be evaluated with smell test
- Management:§
- Replacing LH with hCG
- Replacing FSH with recombinant FSH or hMG, which exhibits both LH and FSH-like activity, after testosterone levels are normalized on hCG
- If medical therapy fails to result in a pregnancy, but some sperm are found in the ejaculate, referral for ART is recommended.
- Prader-Willi syndrome
- Features: hypogonadism, small testes, dysmorphic facies, growth hormone deficiency with short stature, small hands and feet, pain insensitivity, cognitive disorders
Acquired
- Hyperprolactinemia
- See 2018 AUA Testosterone Deficiency Guideline Notes
- Pituitary or suprasellar tumors
- Pituitary infiltrative disorders (e.g., hemochromatosis, tuberculosis, sarcoidosis, histiocytosis)
- Pituitary apoplexy (Sheehan's syndrome)
- Pituitary surgery
- Head trauma
- Exogenous androgens
Testicular
Congenital
- Causes of primary hypogonadism
- DUNKY XX:
- Down syndrone
- Undescended testis/cryptorchidism
- Noonan’s Syndrome
- Klinefelter Syndrome
- Y chromosome micro deletions
- XX-male
- DUNKY XX:
- Klinefelter Syndrome
- Most common known genetic cause of male infertility
- See Disorders of Sexual Differentiation Chapter Notes
- Males with Klinefelter syndrome should be counseled that few non-mosaic XXY men will have sperm in the ejaculate and medically-unassisted paternity is rare.§
- Y chromosome microdeletion
- Second most common known genetic cause of infertility in the male
- Can result from errors that occur during homologous recombination during meiosis due to the palindromic structure of the chromosome
- Majority of (but not all) genes on the Y chromosome encode proteins involved in testis determination or spermatogenesis
- Azoospermia Factor (AZF) region★
- In the long arm of the Y chromosome
- Critical to formation of sperm
- Consists of three areas encoding genes involved in spermatogenesis (AZFa, AZFb, AZFc)
- AZFa (also known as AZF1) or AZFb complete microdeletion: generally result in absence of spermatogenesis.
- Common phenotypic manifestations of deletions in AZFa region are azoospermia and Sertoli cell-only syndrome
- Genes in the AZFb region have been found to support the growth and maturity of sperm and are critical for efficient progression of spermatogenesis. Common phenotypic manifestations of deletions in this region are spermatogenic arrest and azoospermia
- AZFc microdeletion: may result in spermatogenic impairment but not necessarily absence of spermatogenesis
- Genes in the AZFc region have a diverse role, but overall, they are essential to complete spermatogenesis. AZFc deletions have been associated with drastic reduction in sperm count, and there are subsets of men with AZFc microdeletions that experience progressive declines in their sperm count.
- Originally, the AZFb and AZFc genes were identified and thought to be separate regions. They were later found to be overlapping and are now referred to as AZF2.
- AZFa (also known as AZF1) or AZFb complete microdeletion: generally result in absence of spermatogenesis.
- Cryptorchidism
- If underwent orchidopexy and had unilateral cryptorchidism, 96% paternity rate; 70% if bilateral.
- The sooner orchidopexy the better, but age unknown. Better if done prior to age 10.
- Sertoli Cell Only syndrome
- Patients present with normal levels of LH and testosterone. The low level of inhibin-B leads to elevated levels of follicle-stimulating hormone FSH.§
- Leydig cell insufficiency
- Exogenous testosterone not indicated since insufficient testicular testosterone concentrations are achieved for spermatogenesis
- If azoospermia, low testosterone, and elevated LH, perform surgical sperm extraction
- Androgen-receptor (AR) resistance
- See Disorders of Sexual Differentiation Chapter Notes
- Diagnosis and Evaluation: significantly elevated testosterone associated with impaired male fertility. LH is mildly elevated, FSH is normal
Acquired (TICCS)
- Toxins
- Medications
- Associated with infertility
- Finasteride
- 5 mg/day is associated with reduced semen volume, but 1 mg/day data are inconclusive
- Exogenous testosterone/anabolic steroids
- Testosterone is converted to estradiol by aromatase. This estradiol inhibits LH secretion. Consequently, there is decreased intratesticular testosterone synthesis and reduced spermatogenesis.
- Testosterone abuse results in an acquired variant of hypogonadotropic hypogonadism characterized by extremely low or undetectable serum levels of FSH and LH, atrophic testes and severe oligozoospermia or azoospermia.
- Anabolic androgenic steroid abuse was the most frequent cause of profound hypogonadism among young men.
- Injections are the most toxic against spermatogenesis; nasal spray is the least toxic
- While exogenous testosterone does not suppress luteinizing hormone or FSH during puberty in patients with Klinefelter syndrome, testosterone does suppress gonadotropins after puberty.
- Should be ceased as the initial step.
- Majority of men recover fertility in a time-dependent manner.
- Recovery begins on average 4 to 5 months after initiation of medical therapy but it can take up to 2 years
- Probabilities of recovery at 6, 12, 16, and 24 months to be 67%, 90%, 96% and 100%, respectively.
- Older males less likely to recover
- Time to recovery slower in older males, low-normal sperm count prior to starting exogenous testosterone, and with high dose exogenous testosterone.
- Probabilities of recovery at 6, 12, 16, and 24 months to be 67%, 90%, 96% and 100%, respectively.
- Recovery of spermatogenesis can be improved with hCG +/- FSH
- Semen quality will be sufficient for intrauterine insemination in 70% of men within 12 months of medical therapy to promote spermatogenesis
- Low-quality evidence for no impact of anabolic steroids/exogenous testosterone on permanent infertility[3]
- Estrogen
- Anti-androgens
- Finasteride
- Evidence inconclusive★
- Anti-rheumatic medications
- Thiopurines
- Corticosteroids
- Not a risk factor★
- Methotrexate
- Other medications mentioned in Campbell's 11th edition:
- Spironolactone
- HIV medications
- Protease inhibitors (indinavir) and nucleoside reverse transcriptase inhibitors (stavudine)
- Cimetidine
- Sulfasalazine (should be substituted with mesalazine)
- Opioids
- Suppresses LH release resulting in decreased intratesticular testosterone synthesis and reduced spermatogenesis
- Anti-psychotics
- Dopamine antagonists can result in decreased libido
- SSRIs are associated with anorgasmia and delayed or absent ejaculation
- If there is concern about the influence of a particular medication on fertility, clinicians may consult databases with data on reproductive effects of medications such as REPROTOX® for additional information.
- Associated with infertility
- Chemotherapy
- Cancer (especially testicular cancer) can negatively affect spermatogenesis, even before chemotherapy
- It is unknown what duration of time after receiving chemotherapy is needed to have no residual DNA damage
- Sperm DNA damage can be detected at least 2 years after chemotherapy.
- Sperm banking should be prioritized early in the management of a patient with testicular cancer
- Radiation to testes if dose > 7.5 Gy
- Social habits
- Cigarette smoking
- Cannabis decreases plasma testosterone and may affect the acrosome of the spermatozoa§
- Alcohol use
- Drinkers have slightly lower semen volume and slightly poorer sperm morphology, but drinking does not adversely affect sperm concentration or sperm motility[5]
- Caffeine
- Moderate quality evidence of no association (except possibly sperm aneuploidy) between caffeine and male infertility[6]
- Environmental exposure
- Diet
- Poor diet results in reduced fertility
- Stress
- Associated with reduced sperm progressive motility, but has no association with semen volume; data were inconclusive for sperm concentration and sperm morphology[7]
- Medications
- Infections and Inflammation
- History of infections of the GU tract (testis, epididymis, prostate, urethra) is associated with infertility
- Viral orchitis can result in bilateral testicular atrophy
- No effect of HIV or hepatitis
- History of infections of the GU tract (testis, epididymis, prostate, urethra) is associated with infertility
- Childhood
- Hydrocele or hernia surgery can cause obstruction
- Moderate-quality evidence that found the impact of hernia repair on reproductive function to be inconclusive§
- Torsion
- 11% of males develop anti-sperm antibodies after testicular torsion. However, fertility similar to general population
- 2016 study from Israel reviewed torsion 63 patients, 41 with orchiopexy and 22 with orchiectomy, and found that pregnancy rates were similar to general population (≈90%). Mean time to pregnancy approx. 7 months, no difference between orchiopexy and orchiectomy§
- 11% of males develop anti-sperm antibodies after testicular torsion. However, fertility similar to general population
- Hydrocele or hernia surgery can cause obstruction
- Testicular cancer
- Impacts sperm count and concentration, but evidence is inconclusive regarding impact on motility and morphology§
- Increased scrotal temperature
- Scrotal temperature is maintained 2-4°C below body temperature.
- Increasing testis temperature impairs spermatogenesis; safe scrotal temperature is not known
- Age
- Older men have slightly reduced fertility
- All 7 parameters, except concentration, are associated with small age-dependent declines (i.e., semen parameters decrease as age increases)[8]
- Older men have slightly reduced fertility
- Obesity
- Moderately reduced fertility
- Mechanisms related to infertility:
- Adipose tissue, which is the main source of aromatase in men and results in increased E2 levels, lowers T:E2 ratio and increases negative feedback on the HPG axis
- Increased scrotal temperature
- Increased total body surface area, effectively diluting testosterone concentration in testosterone sensitive areas.
- Obesity and metabolic syndrome are associated with an overall increased inflammatory state, suppressing the HPG axis and causing mixed testicular/pituitary hypogonadism.
Post-testicular
- Ejaculatory dysfunction
- Ductal obstruction
- Retrograde ejaculation
- Partial retrograde ejaculation may exist concurrently with partial antegrade ejaculation. If the antegrade specimen is sufficient for reproduction either naturally or with medical assistance, no treatment may be necessary. However, if the antegrade ejaculate is poor and a substantial RE is present as demonstrated by post-ejaculatory urinalysis, various therapies may be required.
- May be treated with ★
- Sympathomimetics and alkalinization of urine with or without urethral catheterization
- Induced ejaculation
- Surgical sperm retrieval
- Anejaculation
- Causes include spinal cord injury, demyelinating neuropathies (multiple sclerosis), diabetes, iatrogenic (RPLND, pelvic surgery) transverse myelitis, and congenital neural tube defects
- Sexual History
- Lubricants impair sperm motility.
- Pre-seed is a lubricant that does not decrease motility.
- Saliva is toxic to sperm
- Lubricants impair sperm motility.
- Other syndromes associated with infertility
- Kartagener syndrome
- Also known as primary ciliary dyskinesia
- Associated with infertility and respiratory pathology due to absence of ciliary movement
- Cystic Fibrosis
- Inheritance: autosomal recessive
- One defective allele must be inherited from each parent for a child to be affected
- Individuals with only one mutation are carriers but do not harbor the disease.
- Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
- Gene responsible for cystic fibrosis
- Regulates anion transport and fluid secretion in the excurrent ducts
- The mutations more likely to cause obstructive azoospermia may be different than those that cause cystic fibrosis[9]
- Characterized by
- Pulmonary obstruction and infection
- Exocrine pancreatic insufficiency
- Obstructive azoospermia
- Dental carries
- Mutations in the CFTR gene can lead to vasal and seminal vesicle agenesis/atresia[10]
- Thought that dysregulation of proper fluid dynamics leads to obstruction and/or atrophy in the epididymis and vas deferens during embryogenesis
- Mutations in the CFTR gene are present in up to
- 80% of men with congenital bilateral absence of the vas deferens (CBAVD)
- 20% of men with CUAVD
- 21% of men with idiopathic epididymal obstruction
- While vasal abnormalities are apparent on physical examination, epididymal obstruction may only be diagnosed at the time of surgical exploration. As such, CFTR testing may necessarily occur after surgical treatment in some men.
- Mutations in the CFTR gene are present in up to
- Not all cystic fibrosis patients have vasal agenesis
- Thought that dysregulation of proper fluid dynamics leads to obstruction and/or atrophy in the epididymis and vas deferens during embryogenesis
- Some men with otherwise idiopathic genital tract obstruction are found to harbor mutations in the CFTR gene
- Inheritance: autosomal recessive
- Kartagener syndrome
Questions
- What is the definition of infertility? What is the definition of primary vs. secondary infertility?
- List causes of male infertility.
Answers
- What is the definition of infertility? What is the definition of primary vs. secondary infertility?
- List causes of male infertility.
Next Chapter: Diagnosis and Evaluation of Infertility
References
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, chap 24
- Khan MA, Pagani RL, Ohlander SJ. 2019 AUA Update: Exogenous Testosterone and Male Reproduction