Upper Urinary Tract Urothelial Cancer
Epidemiology[1]
- Incidence
- Relatively rare
- US incidence: 2/100,000
- Tumors of the renal pelvis are slightly more common than ureteral tumors[2]
- Renal pelvis: 1.2/100,000
- Ureter: 0.8/100,000
- Ureteral tumours occur more commonly in the lower ureter (70%) than in the upper ureter (25% mid, 5% upper). This may be a reflection of downstream implantation.
- Tumors of the renal pelvis are slightly more common than ureteral tumors[2]
- US incidence: 2/100,000
- Higher incidence in Balkan countries
- Relatively rare
- Age
- Peak incidence age 70s and 80s (older than bladder cancer patients)
- Presentation at age <60 should raise concern of hereditary UTUC as part of Lynch syndrome
- Peak incidence age 70s and 80s (older than bladder cancer patients)
- Gender
- M:F 2:1 (unlike bladder which is M:F 4:1)
- Race
- Caucasians are 2x more likely as African-Americans to develop UTUC
Pathogenesis
- Risk factors
- Hereditary
- Lynch syndrome (hereditary nonpolyposis colorectal carcinoma (HNPCC))
- Mutation: DNA mismatch repair genes (MLH1, MSH2, MSH6, and PMS2)
- Associated malignancies (10):
- Colon (most common)
- Endometrial (second most common)
- Prostate
- Urothelial
- Adrenal
- Gastric
- Pancreatic
- Uterine
- Ovarian
- Sebaceous carcinomas
- The urothelial cancers mainly arise in the upper tract, and it is unclear if carriers of these mutations may also have a higher risk of bladder malignancy
- Unlike with non-hereditary cancers, patients are younger (mean age 55 years) and are more likely to be female
- Lynch syndrome (hereditary nonpolyposis colorectal carcinoma (HNPCC))
- External (7)
- Risk factors shared with bladder cancer (5)
- Smoking
- Cigarette smoking is the most important modifiable risk factor for UTUC
- Smoking cessation decreases subsequent risk
- Occupational exposure
- Exposure to aromatic hydrocarbons, especially those used in the chemical, petrol, and plastic industries; other occupations at risk are those with exposure to coal, asphalt, or tar
- Chronic inflammation, infection, or iatrogenesis
- Chronic bacterial infection associated with urinary stones and obstruction has been associated with the development of squamous cell cancer (and less commonly adenocarcinoma)
- Alkylating chemotherapy
- Cyclophosphamide or ifosfamide
- Analgesic abuse
- Phenacetin is the most well described causative agent in analgesic nephropathy, others include codeine, acetaminophen, and aspirin.
- The number of cases attributed to phenacetin has decreased since phenacetin was replaced by its non-toxigenic metabolite acetaminophen
- Smoking
- Unique to UTUC (2):
- Aristolochic acid
- Found in plants (Aristolochia fangchi and Aristolochia clematitis) and has mutagenic action; the associated mutation is predominant in patients with Balkan endemic nephropathy and Chinese herb nephropathy. These plants are endemic in Balkan countries and grow as weeds in wheat fields. The incidence of Balkan endemic nephropathy is decreasing.
- Arsenic
- Exposure can be from drinking water from artesian wells
- Aristolochic acid
- Risk factors shared with bladder cancer (5)
- Hereditary
Pathology
- Normal upper tract urothelium
- Bladder is derived from the endoderm; ureter and renal pelvis are derived from the mesoderm
- The urothelial lining of the upper urinary tract closely approximates that of the bladder except for the markedly reduced thickness of the muscle layer and the abutting of the urothelium to the renal parenchyma proximally.
- The epithelial layer is continuous from the level of the calyces to the distal ureter.
- It has been postulated that the urothelial layer may even “extend” into the collecting ducts, raising the possibility that collecting duct renal cancers may be closely related to urothelial cancers and perhaps better treated by agents used for urothelial cancers
- Renal pelvis and calyces
- The walls of the calyces and the pelvis contain fibrous connective tissue and 2 layers of smooth muscle and are lined on their inner surfaces with urothelium
- Ureter
- The 3 muscular layers of the ureter merge with the 3 muscular layers of the bladder
- Abnormal urothelium
- Benign lesions
- Papillomas and inverted papillomas
- Generally considered benign lesions
- Association with either synchronous or metachronous UTUC
- Follow-up for all cases of inverted papilloma should be continued for at least 2 years after initial diagnosis
- Von Brunn Nests
- Reactive proliferation, considered a variation of normal urothelium.
- Metaplasia and dysplasia
- In a significant proportion of patients, UTUCs progress from hyperplasia to dysplasia to frank CIS
- Urothelial carcinoma
- Majority (90%) of upper tract tumours are urothelial carcinoma[3]
- Squamous and adenocarcinomas comprise a small minority.
- UTUC are histologically similar to urothelial carcinoma of the bladder, but the relative thinness of the muscle layer of the renal pelvis and ureter may allow earlier penetration of invasive upper tract tumors than is seen in bladder neoplasms.
- UTUC is more often invasive and poorly differentiated than bladder cancers. However, in pathologically matched cohorts, cancer-specific outcomes are comparable between urothelial tumours of the upper tract and bladder
- Reported variants of urothelial carcinoma are squamous cell, glandular, sarcomatoid, micropapillary, neuroendocrine, and lymphoepithelial.
- Although all of these variants are considered aggressive tumors, after adjustment for the rest of clinicopathologic characteristics, variant histology has not been shown to predict poor clinical outcome in UTUC (unlike bladder cancer)
- Non-urothelial carcinoma
- Most commonly squamous cell carcinoma and adenocarcinoma
- Squamous
- Frequently associated with a condition of chronic inflammation or infection or with analgesic abuse
- Typically more aggressive at presentation
- Occur 6x more frequently in the renal pelvis than in the ureter
- Adenocarcinoma
- Rare
- Typically associated with long-term obstruction, inflammation, or urinary calculi
TNM staging (AJCC 8th edition)
- Tstage
- TX: tumour cannot be assessed
- T0: no evidence of tumour
- Ta: non-invasive (confined to epithelial mucosa) papillary carcinoma
- Tis: carcinoma in-situ
- T1: invades lamina propria (subepithelial connective tissue)
- T2: invades muscle
- T3
- Renal pelvis: tumour invades beyond muscularis into peripelvic fat or renal parenchyma
- Ureter: tumour invades beyond muscularis into periureteric fat
- T4: invades adjacent organs or through the kidney into perinephric fat
- Nstage
- NX: regional lymph nodes cannot be assessed
- N0: no regional lymph node metastasis
- N1: metastasis ≤2 cm in greatest dimension, in a single lymph node
- N2: metastasis >2 cm in a single lymph node; or multiple lymph nodes
- Mstage
- MX: distant metastasis cannot be assessed
- M0: no distant metastasis
- M1: distant metastasis
Natural history
- Most occurrences are in a single renal unit
- Synchronous bilateral UTUC
- Rare (<2%)
- Risk of bilateral disease and multifocality increases with the presence of CIS
- Metachronous UTUC occurrences are 80% after bladder cancer and 2-6% after contralateral UTUC
- 2-4% patients with bladder cancer will subsequently develop UTUC
- Interval ranges from 17-170 months
- Risk factors for subsequent UTUC in patients undergoing cystectomy for bladder cancer (9)
- Low-grade tumors
- Non–muscle invasive tumors
- N0 status
- Presence of CIS
- Multiple urothelial recurrences
- Multifocal tumors
- History of previous UTUC
- Positive ureteral margin
- Involvement of male prostatic urethra or female urethra
- Based on meta-analysis of 27 studies with 13,185 patients Picozzi et al. 2012
- Long-term surveillance of the upper tract is important in bladder cancer patients
- ≈30% patients with UTUC will subsequently develop bladder cancer after nephroureterectomy or nephron-sparing procedures
- Median time to bladder recurrence 6-12 months
- Given the high incidence of metachronous bladder involvement, routine bladder surveillance should be performed in patients with a history of UTUC
- Potential explanations on why bladder cancers following UTUC are more common than UTUC following bladder cancer include:
- Downstream seeding
- Longer exposure time to carcinogens in the bladder
- Greater number of urothelial cells in the bladder that are subject to random carcinogenic events
- UTUC may spread in the same ways as bladder tumors do via direct invasion into the renal parenchyma or surrounding structures, lymphatic or hematogenous invasion, and epithelial spread by seeding or direct extension.
- Lymphatic:
- Lymphatic spread from the upper urinary tract depends on the location of the tumor:
- Renal pelvis and upper ureteral tumors spread initially from hilar to para-aortic and paracaval nodes
- Distal ureteral tumors spread to pelvic nodes
- Risk of lymphatic spread is directly related to the depth of invasion (stage) of the primary tumor
- Hematogenous:
- Most common sites of hematogenous metastases: liver, lung, and bone
- Epithelial:
- Epithelial spreading may occur in both antegrade and retrograde manners.
- Antegrade seeding is more common and thought to be the most likely explanation for the high incidence of recurrence in patients in whom a ureteral stump is left in situ after nephrectomy and incomplete ureterectomy
- Panurothelial disease
- Defined as a disease involving the bladder and 2 extravesical sites
- In males, this could include one or both upper urinary tracts and/or the prostatic urethra
- In females, this could be the bladder and both upper urinary tracts.
- The low incidence and the lack of prospective studies do not permit absolute conclusions about treatment impact and outcomes
Diagnosis and Evaluation of UTUC
- UrologySchool.com Summary
- H+P
- Labs: cytology, LFTs
- Imaging:
- Primary: CT urography, retrograde imaging
- Metastasis: CXR
- Other:
- Cystoscopy
- URS +/- biopsy
- Renal mass biopsy
- History and physical exam
- Most common presenting sign is hematuria
- Flank pain is the second most common symptom.
- This pain is typically dull and believed to be secondary to a gradual onset of obstruction and hydronephrotic distention. In some patients, pain can be acute and can mimic renal colic, typically due to the passage of clots that acutely obstruct the collecting system.
- Some patients are asymptomatic at presentation and are diagnosed when an incidental lesion is found on imaging
- Laboratory
- Cytology and other markers
- The sensitivity of cytology is directly related to tumor grade
- In a patient with an upper tract filling defect and an abnormal voided cytology, must be cautious in determining the site of origin of the malignant cells. Ureteral catheterization for collection of urine or washings may provide more accurate cytologic results.
- Causes of false-positive cytology (2):
- Contrast agents
- Exposure of urothelial cells to ionic, high-osmolar contrast agents as in retrograde pyelography may worsen cytologic abnormalities.
- Clinical implication: cytologic specimens should be obtained before the use of these agents
- Inflammation from infection or stones
- Liver function tests
- Liver is a common site of metastasis
- Imaging
- Primary
- Options:
- Retrograde ureterogram and pyelogram
- CT urography
- High sensitivity (100%) and moderate specificity (60%) for upper tract malignant disease
- Options:
- Primary

- Typical findings suggestive of an upper urinary tract tumor:
- Radiolucent filling defects
- Non-visualization of the collecting system
- Obstruction
- Differential diagnosis of a radiolucent filling defect (7):
- Tumour
- Blood clot
- Stones; higher HFU than urothelial carcinoma
- Sloughed papilla
- Fungus ball
- Overlying bowel gas
- External compression
- Radiolucent, noncalcified lesions may require additional evaluation by retrograde urography or ureteroscopy, with or without biopsy and cytology
- Evaluation of the contralateral kidney is important not only because of possible bilateral disease but also because it allows a determination of the functionality of the contralateral kidney
- Metastasis
- CXR
- Bone scan, consider in the presence of bone pain, elevated calcium or elevated alkaline phosphatase
- Other
- Cystoscopy
- Because upper urinary tract tumors are often associated with bladder cancers, cystoscopy is mandatory in the evaluation to exclude coexistent bladder lesions
-
URS with biopsy
- Diagnostic accuracy improves with the addition of URS to retrograde imaging
- Cystoscopy
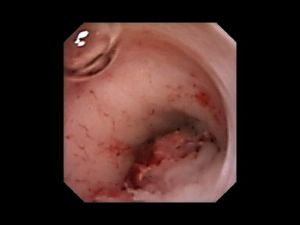
Ureteral tumour on endoscopy
- URS allows direct visualization of the tumor and biopsy of suspected areas
- The urologist’s impression of the tumor grade based on ureteroscopic appearance is likely to be correct in only 70% of cases, suggesting that biopsy is also needed to further define this important aspect of staging
- Systematic review and meta-analysis evaluating diagnostic accuracy of URS biopsy (2020)
- Included studies comparing URS biopsy to pathology on surgical specimen (radical nephroureterectomy or segmental ureterectomy)
- Results:
- Included 23 studies comprising 2232 patients
- Moderate to high risk of bias accross studies
- Stage-to-stage match
- Positive predictive value for cT1+/muscle-invasive: 94%
- Negative predictive value for cTa-Tis/non-muscle-invasive disease of 60%
- Grade-to-grade match
- High-grade (cHG/pHG): 97%
- Low-grade (cLG/pLG): 66%
- Grade-to-stage match
- Positive predictive value for cHG/muscle-invasive disease: 60%
- Negative predictive value for cLG/non-muscle-invasive disease: 77%
- Overall
- 32% undergrading
- 46% understaging
- A precise correlation with eventual tumor stage is difficult mainly because of technical limitations of use of small biopsy instruments through the narrow channel of the flexible ureteroscope, resulting in the small size and shallow depth of ureteroscopic biopsy specimens. Brush biopsy may be used if cup biopsy forceps fail to obtain adequate tissue.
- Subiela, José Daniel, et al. "Diagnostic accuracy of ureteroscopic biopsy in predicting stage and grade at final pathology in upper tract urothelial carcinoma: Systematic review and meta-analysis." European Journal of Surgical Oncology (2020).
- Reasonable histologic correlation (78-92%)
- In general, CIS of the upper tract is a presumptive diagnosis that is made by the presence of unequivocally positive selective cytology in the absence of any radiographic or endoscopic findings
- Is URS (with or without biopsy) necessary in all cases of suspected upper tract tumors? No. In fact, URS should probably be reserved for situations in which the diagnosis remains in question after conventional radiographic studies and for those patients in whom the treatment plan may be modified on the basis of the ureteroscopic findings, for example, endoscopic resection.
- Technique: Endoscopic Evaluation and Collection of Urine Cytology Specimen
- Summary of Steps
- Cystoscopy is performed and the bladder inspected for concomitant bladder disease.
- The ureteral orifice is identified and inspected for lateralizing hematuria.
- A small-diameter (6.9 or 7.5 Fr) ureteroscope is passed directly into the ureteral orifice, and the distal ureter is inspected before any trauma from a previously placed guidewire or dilation.
- A guidewire is then placed through the ureteroscope and up the ureter to the level of the renal pelvis under fluoroscopic guidance.
- The flexible ureteroscope is used to visualize the remaining urothelium.
- When a lesion or suspicious area is seen, a normal saline washing of the area is performed before biopsy or intervention. If the ureter does not accept the smaller ureteroscope, active dilation of the ureter is necessary.
- Special circumstances include prior urinary diversion and tumor confined to the intramural ureter. With cases of prior urinary diversion, identification of the ureteroenteric anastomosis is difficult and may require antegrade percutaneous passage of a guidewire down the ureter before endoscopy. The wire can be retrieved from the diversion, and the ureteroscope can be passed in a retrograde fashion. The nephrostomy tract does not need to be fully dilated in this setting
- Summary of Steps
- Antegrade endoscopy
- Percutaneous access to the renal pelvis may be required for diagnosis or treatment. In such cases, antegrade urography and ureteroscopy may be useful for tumor resection, biopsy, or simple visualization.
- Tumor cell implantation in the retroperitoneum and along the nephrostomy tube tract has been reported after these procedures
-
Renal mass biopsy
- Systematic review of 288 patients undergoing percutaneous nephroscopic resection of tumour found a tract seeding rate of 0.3%[4]
- Preoperative determination of the stage of UTUC tumors remains difficult. Therefore, in predicting the tumor stage, a combination of the radiographic studies, the visualized appearance of the tumor, and the tumor grade provides the surgeon with the best estimation for risk stratification.
Prognosis
- Tumour factors
- Stage
- Most important prognostic factor
- Non–organ confined disease (>pT2) is the most significant predictor of the development of metastases
- Most important prognostic factor
- Grade
- High-grade tumours are more likely to:
- Invade into the underlying connective tissue, muscle, and surrounding tissues
- Be associated with concomitant CIS
- While there is strong correlation between stage and grade, each independently predicts post-operative recurrence
- High-grade tumours are more likely to:
- Architecture
- ≈85% of renal pelvic tumors are papillary and the remainder sessile
- Papillary tumors seem to have better outcomes than sessile lesions
- Invasion of the lamina propria or muscle (stage T1 or T2) occurs in 50% of papillary and in >80% of sessile tumors
- CIS of the upper tract is associated with higher risk for disease progression (similar to bladder cancer) and a likelihood of future development of invasive urothelial cancers.
- Size
- Tumours > 3-4 cm may be associated with worse survival as well as a higher risk of bladder recurrence
- Location
- Renal pelvic tumours are usually more aggressive than ureteral tumours
- 50-60% of renal pelvic tumors are invasive into either the lamina propria or muscle, in contrast to most bladder tumors, which are usually non-invasive
- 55-75% of ureteral tumors are low grade and low stage, but invasion is still more common than bladder tumors
- The renal parenchyma may be a barrier, slowing distant spread of stage T3 renal pelvis tumors. In contrast, periureteral tumor extension carries a high risk of early tumor dissemination along the periureteral vascular and lymphatic supply. Improved survival of patients with stage T3 renal pelvis tumors versus ureteral tumors has been reported
- Conflicting results on whether the location of an upper tract tumor affects prognosis
- Renal pelvic tumours are usually more aggressive than ureteral tumours
- Multifocality
- Defined as presence of tumor in ≥2 sites within urothelium
- Independent predictor of poor clinical outcome
- Tumour necrosis
- Conflicting evidence on the influence of tumour necrosis on survival
- Lymph node involvement
- Although lymphadenectomy is seldom performed for clinically node-negative disease, pathologic lymph node status is a strong predictor of post-nephroureterectomy recurrence
- LVI
- Associated with worse survival in patients without positive nodes; no association in N+ disease
- Hydronephrosis
- Independently associated with advanced disease stage and poor survival
- Positive surgical margins
- Previous or concomitant bladder tumours
- Stage
- Patient factors
- Age
- Increasing age associated with worse survival
- Race
- Black non-Hispanic race is associated with increased mortality
- Age
- Surgical factors
- Lack of post-operative mitomycin C instillation
- 2021 EAU risk-stratification in UTUC:
- Low-risk (5):
- Unifocal disease
- Tumour size < 2 cm
- Negative for high-grade cytology
- Low-grade URS biopsy
- No invasive aspect on CT
- High-risk (8):
- Multifocal disease
- Tumour size ≥ 2 cm
- High-grade cytology
- High-grade URS biopsy
- Hydronephrosis
- Previous radical cystectomy for high-grade bladder cancer
- Variant histology
- Local invasion on CT
- Low-risk (5):
- Clinical prediction tools have been developed for risk stratification before and after definitive therapy
- 3 particular forms of UTUC, 2 associated with environmental exposure (aristolochic acid nephropathy, which includes Balkan and Chinese herbal nephropathy, as well as those seen in arsenic-endemic regions), analgesic abuse, and those associated with Lynch syndrome, have an even higher tendency have multiple and bilateral recurrences than do sporadic tumors
- 5-year overall survival rates:
- Grade
- 1-2: 40-87%
- 3-4: 0-33%
- Stage
- Ta, T1, CIS: 60-90%
- T2: 43-75%
- T3: 16-33%
- T4: 0-5%
- N+: 0-4%
- M+: 0%
Management
- As of April 2021, there were only EAU guidelines on Upper Tract Urothelial Carcinoma. None available from AUA, CUA, or NCCN.
- Non-metastatic disease
- Options:
- Radical nephroreterectomy with bladder cuff excision
- Nephron-sparing approaches
- Endoscopic ablation/resection
- Ureteroscopic
- Percutaneous
- Segmental ureterectomy
- Intraluminal therapy
- Endoscopic ablation/resection
- Options:
-
EAU Guideline Recommendations based on risk-stratification (see above)
-
Low-risk
- Nephron-sparing approaches recommended for ALL low-risk UTUC, irrespective of the status of the contralateral kidney
- Reduces morbidity associated with radical surgery without compromising oncological outcomes
- Note that 2013 CUA Upper Tract Surveillance Guidelines described radical nephrouretectomy with bladder cuff excision as the gold standard management for non-metastatic UTUC
- Radical nephroureterectomy with bladder cuff excision may be considered for large, multifocal, or rapidly recurring tumours, or tumours that have failed maximal efforts are conservative surgery
- High-risk
- Radical nephrouretectomy with bladder cuff excision +/- chemotherapy
- Also used for low-grade, non-invasive tumors of the renal pelvis and upper ureter when they are large, multifocal, or rapidly recurring despite maximal efforts at conservative surgery
- Nephron-sparing approaches may be considered in select patients with:
- Renal insufficiency (functionally or anatomically)
- Radical nephroureterectomy and dialysis still offer the best chance of cure and survival in patients with a large, invasive, high-grade, organ-confined renal pelvis tumor (T2N0M0) in a solitary kidney.
- For a patient with significant life expectancy, the risks and morbidity on hemodialysis are less than the risks of an inadequately treated aggressive high-grade UTUC.
- Radical nephroureterectomy and dialysis still offer the best chance of cure and survival in patients with a large, invasive, high-grade, organ-confined renal pelvis tumor (T2N0M0) in a solitary kidney.
- Pre-disposition to multiple recurrences
- Renal insufficiency (functionally or anatomically)
- In patients with radiographic suspicion for lymph node involvement, the current paradigms are shifting toward primary treatment with platinum-based neoadjuvant chemotherapy with surgical consolidation offered only if a significant response is seen
- Radical nephrouretectomy with bladder cuff excision +/- chemotherapy
-
- Nephron-sparing approaches (3):
- Endoscopic
- Segmental ureterectomy
- Intraluminal therapy
- Systematic review and meta-analysis comparing nephon-sparing approach to radical nephroureterectomy (2016)
- Primary outcome: cancer-specific survival
- Results
- Included 22 studies published between 1999 and 2015
- No RCTs comparing nephron-sparing approach and nephroureterectomy
- High risk of bias across all domains analysed, limiting interpretation of comparisons
- Segemental ureterectomy vs. RNU (10 studies): no significant difference in cancer-specific survival
- Endoscopic vs. RNU
- URS vs. RNU (5 studies): no significant difference in cancer-specific survival
- Grade-based subgroup analyses found decreased cancer-specific survival in patients undergoing URS for high-grade disease
- Percutaneous resection vs. RNU (2 studies): conflicting findings
- URS vs. RNU (5 studies): no significant difference in cancer-specific survival
- Included 22 studies published between 1999 and 2015
- Seisen, Thomas, et al. "Oncologic outcomes of kidney-sparing surgery versus radical nephroureterectomy for upper tract urothelial carcinoma: a systematic review by the EAU non-muscle invasive bladder cancer guidelines panel." European urology 70.6 (2016): 1052-1068.
-
Endoscopic Treatment
-
Indications
-
Low-grade, non-invasive tumours that can be completely resected with this approach
- Patient's with unresectable, high grade, or invasive should proceed immediately to nephroureterectomy, provided they are medically fit.
-
- Outcomes
- Systematic review of endoscopic management of UTUC (2012)
- Results:
- Included 34 studies, 22 on URS and 12 on percutaneous resection
- All were case series (level of evidence 4), or non-randomised comparative studies (level of evidence 3b)
- Mean sample size
- URS: 33
- PCN: 24
- 3 institutions for URS and 1 for PCN, have published outcomes on cohorts of ≥ 40 patients with > 50 months follow-up, limiting generalzability
- Estimated 5-yr:
- Recurrence-free survival: 13 – 54%
- Renal preservation: 85%
- Cancer-specific survival: 49-89%
- OS 57-75%
- Recurrence-free and cancer-specific survival outcomes worsened with increasing grade
- Cutress, Mark L., et al. "Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review." BJU international 110.5 (2012): 614-628.
- Included 34 studies, 22 on URS and 12 on percutaneous resection
- Results:
- Given high risk of recurrence with endoscopic management, patients should be informed of the need for early second-look and stringent surveillance.
- Risk of disease progression remains with endoscopic management due to the suboptimal performance of imaging and biopsy for risk stratification and tumour biology
- Systematic review of endoscopic management of UTUC (2012)
- Approach
- Retrograde (ureteroscopic) vs. antegrade (percutaneous)
- Choice depends largely on the tumor location and size
- Retrograde preferred when tumor size, number, and access allow complete tumor ablation.
- Percutaneous antegrade tumor ablation preferred when the anatomy and the tumor do not allow complete ablation through a retrograde approach because of location (e.g., lower pole calyx or proximal ureter) or previous urinary diversion
- In cases with multifocal involvement, combined antegrade and retrograde approaches can be considered
- Choice depends largely on the tumor location and size
- Retrograde approach
- Advantages (2):
- Lower morbidity than percutaneous and open surgical counterparts
- Maintenance of a closed system
- Non-urothelial surfaces are not exposed to the possibility of tumor seeding
- Disadvantages (2):
- Smaller instruments required
- Smaller endoscopes have a smaller field of view and working channel which limits the size of tumor that can be approached in a retrograde fashion. These small instruments limit the accuracy of biopsies, especially with regard to staging
- Some portions of the upper urinary tract, such as the lower pole calyces, cannot be reliably reached with working instruments.
- Smaller instruments required
- Technique
- The tumor is debulked by use of either biopsy forceps or a flat wire basket engaged adjacent to the tumor. Next, the tumor base is treated with either electrocautery or laser energy sources. This technique is especially useful for low-grade papillary tumor on a narrow stalk. The specimen is sent for pathologic evaluation.
- Alternatively, a ureteroscopic resectoscope is used to remove the tumor. Only the intraluminal tumor is resected, and no attempt is made to resect deep (beyond the lamina propria).
- Electrocautery delivered through a small Bugbee electrode (2 or 3 Fr) can be used to fulgurate tumors. However, the variable depth of penetration can make its use in the ureter dangerous, and circumferential fulguration should be avoided because of the high risk of stricture formation. More recently, laser energy with either a neodymium:yttrium-aluminum-garnet (Nd:YAG) or a holmium:YAG (Ho:YAG).
- A ureteral stent is placed for a variable duration to aid with the healing process.
- Advantages (2):
- Antegrade approach
- Advantages (4):
- Ability to use larger instruments that can remove a large volume of tumor in any portion of the renal collecting system
- Improved tumour staging and grading because deeper biopsy specimens are obtained
- May avoid the limitations of flexible ureteroscopy, especially in complicated calyceal systems or areas difficult to access, such as the lower pole calyx or the upper urinary tract of patients with urinary diversion.
- The established nephrostomy tract can be maintained for immediate postoperative nephroscopy and administration of topical adjuvant therapy
- Disadvantages (4):
- Increased morbidity compared with ureteroscopy
- Risk of nephrostomy tract insertion
- Procedure usually requires inpatient admission
- Potential for tumor seeding outside the urinary tract; tract seeding is a possibility but appears to be an uncommon event
- Advantages (4):
- Technique [Further details in Campbell’s]
- Regardless of approach, a nephrostomy tube is left in place. This access can be used for second-look follow-up nephroscopy to ensure complete tumor removal. Follow-up (second-look) nephroscopy is performed 4-14 days later to allow adequate healing. The tumor resection site is identified, and any residual tumor is removed.
- Complications from percutaneous management of tumors are similar to those for benign renal processes and include bleeding, systemic absorption of hypo-osmotic irrigation (with monopolar resection), perforation of the collecting system, and secondary ureteropelvic junction obstruction.
- Retrograde (ureteroscopic) vs. antegrade (percutaneous)
-
- Segmental ureterectomy
- Indications (2):
- Low-grade, low-stage tumors that are not able to be removed endoscopically because of tumor size or multiplicity
- High-grade or invasive tumors when preservation of renal unit is necessary
- Outcomes
- Systematic review and meta-analysis comparing segmental resection to radical nephroureterectomy (2020)
- Results:
- Included 18 studies comprising 4797 patients, of which 1313 underwent segementsl resection
- High risk of bias across all domains analysed, limiting interpretation of comparisons
- 5-yr:
- Recurrence-free survival: significantly worse with segemental resection
- Cancer-specific survival: no significant difference
- OS: no significant difference
- Veccia, Alessandro, et al. "Segmental ureterectomy for upper tract urothelial carcinoma: a systematic review and meta-analysis of comparative studies." Clinical genitourinary cancer 18.1 (2020): e10-e20.
- Results:
- The documented risk of wound implantation by tumor is low after open segemental ureterectomy if simple precautions are followed to minimize spillage
- Segmental ureterectomy of the proximal two-thirds of ureter is associated with higher failure rates than for the distal ureter.
- Systematic review and meta-analysis comparing segmental resection to radical nephroureterectomy (2020)
- Technique
- See Segmental Ureterectomy Chapter Notes for technical aspects
- Indications (2):
- Intraluminal therapy
- Used in 3 settings for treatment of UTUC:
- Primary treatment for CIS
- Adjuvant therapy after endoscopic or organ-sparing therapy
- Primary treatment of low-grade UTUC (UGN-101)
- Management of Positive Upper Tract Urinary Cytology or Carcinoma in Situ
- Diagnosis and Evaluation
- First, repeat the cytology to confirm the findings
- Any source of inflammation, such as urinary infection or calculus, may produce a false-positive result
- A subsequent cytologic abnormality from the contralateral side during follow-up is not rare in cases of true-positive results from early CIS
- Next, radiographic evaluation of the upper tracts, usually with CT urography, and a complete bladder evaluation with cystoscopy
- If the bladder evaluation was
- Positive for bladder tumour, treat the bladder and follow the voided urinary cytologies.
- If cytology remains positive despite a negative bladder evaluation or after successful treatment of the bladder, proceed to evaluating extravesical sites.
- Negative for bladder tumour, evaluate extravesical sites.
- Evaluation of extravesical sites should include selective cytologies from each upper urinary tract, ensuring non-contamination of the specimen from the bladder or urethra, as well as resection of a representative specimen of the prostatic urethra in men.
- Selective cytologies should preferably be done, along with ureteroscopy, to allow for direct visualization of the upper urinary tracts.
- In cases of unilateral upper tract cytologic abnormalities (with normal cystoscopy, pyelography, and bladder biopsies), ureteropyeloscopy is indicated as the next step.
- Ureteropyeloscopy allows for direct visualization of small lesions and is superior to retrograde pyelography in the detection of small tumors.
- Biopsy at the time of ureteropyeloscopy should be attempted, if feasible. A persistently abnormal cytology without any visualized lesions may signify CIS.
- Evaluation of extravesical sites should include selective cytologies from each upper urinary tract, ensuring non-contamination of the specimen from the bladder or urethra, as well as resection of a representative specimen of the prostatic urethra in men.
- Positive for bladder tumour, treat the bladder and follow the voided urinary cytologies.
- If the bladder evaluation was
- First, repeat the cytology to confirm the findings
- CIS of the Upper Urinary Tracts
- In most cases, the diagnosis is one of exclusion wherein there is a persistent positive selective cytology in the absence of any ureteroscopic or radiographic findings.
- The diagnosis of CIS of the upper urinary tracts difficult because of the inability to evaluate the urothelium of the upper tracts with adequate tissue samples
- Management
- Not well established
- Current approaches for presumed upper tract CIS include t opical immunotherapy or chemotherapy
- Most experience is from use of BCG via a nephrostomy tube for primary treatment of CIS.
- Systematic review and meta-analysis evaluating intraluminal therapy for UTUC (2019)
- Inclusion criteria: studies evaluating patients with upper tract urothelial carcinoma receiving instillation treatment as adjuvant/curative therapy for pTa/pT1 and CIS, respectively.
- Studies with ≥10 participants included in quantitative analyses
- Results
- Included 226 patients from 15 studies of patients that underwent BCG instillation for CIS
- Recurrence-free survival: 84%
- Cancer-specific survival: 34%
- Overall survival: 16%
- No difference in survival based on approach (antegrade, retrograde, or combined) or drug (MMC vs. BCG)
- Foerster, Beat, et al. "Endocavitary treatment for upper tract urothelial carcinoma: a meta-analysis of the current literature." Urologic Oncology: Seminars and Original Investigations. Vol. 37. No. 7. Elsevier, 2019.
- Systematic review and meta-analysis evaluating intraluminal therapy for UTUC (2019)
- Historically, radical nephroureterectomy was performed for a unilateral cytologic abnormality of the upper tract to eliminate presumed CIS. This practice is not recommended in the absence of any histologic, radiographic, or endoscopic finding owing to the limitations of cytology alone with false-positive results and the high risk for bilateral disease in the future
- Observation is also not appropriate without further evaluation given the repeated abnormal cytologies.
- Most experience is from use of BCG via a nephrostomy tube for primary treatment of CIS.
- Another scenario is CIS of ureteral margins during radical cystectomy. There is controversy over the management of this finding
- In most cases, the diagnosis is one of exclusion wherein there is a persistent positive selective cytology in the absence of any ureteroscopic or radiographic findings.
- Diagnosis and Evaluation
- UGN-101 (also known as Mitogel, Jelmyto)
- OLYMPUS (Lancet Onc 2020)
- Objective: evaluate the safety and activity of UGN-101 to treat primary and recurrent low-grade UTUC.
- Design: open-label, single-arm, phase 3 trial
- Population: 71 patients with primary or recurrent biopsy-proven, low-grade UTUC (involving the renal pelvis or calyces) and ≥1 low-grade lesion above the ureteropelvic
junction, measuring 5–15 mm.
- Lesions >15 mm were eligible for endoscopic downsizing before the initiation of treatment.
- Treatment: 6 once-weekly treatments of UGN-101
- Primary outcome: complete response, defined as
- Negative endoscopic examination AND
- Negative cytology at the primary disease evaluation AND
- Negative for-cause biopsy when done
- Results:
- Primary outcome:
- 59% complete response at 3 months
- 48% complete response at 12 months[5]
- Adverse events
- Common; 94% had any adverse event
- 37% had ≥1 serious adverse event
- 43% ureteric stenosis
- 20% renal dysfunction
- Primary outcome:
- Kleinmann, Nir, et al. "Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial." The lancet oncology 21.6 (2020): 776-785.
- OLYMPUS (Lancet Onc 2020)
- Approaches:
- Antegrade via percutaneous nephrostomy
- Retrograde through a single J open-ended ureteric stent
- Suboptimal because the drug often does not reach the renal pelvis
- Both the antegrade and retrograde approach can be dangerous due to possible ureteric obstruction and consecutive pyelovenous influx during instillation/perfusion.
- Used in 3 settings for treatment of UTUC:
- Radical nephroreterectomy with bladder cuff excision
- Outcomes largely dependent on clinicopathologic characteristics.
- See Nephroureterectomy Chapter Notes for technical aspects
- Lymphadenectomy
- Prospective studies are needed to assess the role of lymphadenectomy in UTUC.
- There may be prognostic and therapeutic value in patients with invasive disease (T2 to T4), and extended lymphadenectomy is beneficial for accurate staging.
- Adjuvant therapy
- After nephron-sparing therapy
- Nephron-sparing approaches are associated with high risk of local recurrence; patients need to be followed vigilantly for disease progression.
- Options:
- Intraluminal/instillation therapy (immuno- or chemotherapy)
- Brachytherapy of the nephrostomy tract
- Intraluminal therapy
- Treatment agents include thiotepa, mitomycin, and BCG
- Systematic review and meta-analysis (2019)
- Inclusion criteria: studies evaluating patients with upper tract urothelial carcinoma receiving instillation treatment as adjuvant/curative therapy for pTa/pT1 and CIS, respectively.
- Studies with ≥10 participants included in quantitative analyses
- Results
- Included 212 patients from 12 studies of patients that underwent endoscopic laser ablation and instillation therapy for Ta/T1 UTUC
- Recurrence-free survival: 40%
- Similar to recurrence-free survival with observation after nephron-sparing surgery
- Cancer-specific survival: 94%
- Overall survival: 71%
- No difference in survival based on approach (antegrade, retrograde, or combined) or drug (MMC vs. BCG)
- Foerster, Beat, et al. "Endocavitary treatment for upper tract urothelial carcinoma: a meta-analysis of the current literature." Urologic Oncology: Seminars and Original Investigations. Vol. 37. No. 7. Elsevier, 2019.
- The most common complication of intraluminal/instillation therapy is bacterial sepsis
- Brachytherapy
- Brachytherapy to the nephrostomy tract through iridium wire or delivery system has been described
- After complete excision
- Adjuvant radiation
- Radical nephroureterectomy alone provides a high rate of local control; adjuvant radiation without chemotherapy for high-stage disease does not protect against a high rate of distant failure
- Retrospective studies suggest that there may be a role for combined radiation-chemotherapy regimens in patients with advanced disease with adverse features
- Adjuvant chemotherapy
- Intravesical
- ODMIT-C (O'Brien et al. European Urology 2011)
- Population: 284 patients with no previous or concurrent history of bladder cancer undergoing nephroureterectomy for suspected UTUC
- Randomized to a single postoperative intravesical dose of MMC (40 mg in 40 ml saline) at the time of urinary catheter removal vs. standard management
- Primary outcome: incidence of bladder cancer in the first 12 months following nephroureterectomy
- Results:
- Risk of bladder tumour in first 12 months reduced by ≈10% (27% MMC vs. 17% standard treatment, p=0.055 modified intention-to-treat, p=0.03 per-protocol)
- O'Brien, Tim, et al. "Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial)." European urology 60.4 (2011): 703-710.
- Unknown efficacy in patients with previous or concurrent history of bladder cancer
- Unknown whether recurrences that are reduced are low-grade, low-stage lesions or more aggressive ones
- ODMIT-C (O'Brien et al. European Urology 2011)
- Systemic
- The use of agents for UTUC has been extrapolated from chemotherapy regimens used in bladder urothelial cancer
- Neoadjuvant
- Lack of trials that establish the efficacy of neoadjuvant chemotherapy for UTUC.
- Adjuvant
- POUT (Lancet 2020)
- Population: 260 patients with histologically confirmed pT2-T4, N0-3, M0 or pTany, N+, MO UTUC
- Randomized to 4 cycles of gemcitabine-cisplatin (gemcitabine-carboplatin if GFR 30-49ml/min) or surveillance with subsequent chemotherapy, if required
- Primary outcome: disease-free survival
- Secondary endpoints included metastasis-free survival, overall survival, toxicity & quality of life
- Results
- Trial closed early as data met early stopping rule for efficacy
- Median follow-up: 30 months
- Disease-free survival improved by 21% at 3 years (71% chemotherapy vs. 46% surveillance; HR 0.45)
- Significantly improved metastasis-free survival; OS data not mature
- Toxicity: neutropenia, thrombocytopenia, nausea, febrile neutropenia, vomiting; QOL worse initially with chemotherapy, similar by 6 months
- Birtle, Alison, et al. "Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial." The Lancet (2020).
- A disadvantage of adjuvant chemotherapy is that many patients have baseline chronic kidney disease, which worsens after nephroureterectomy, rendering them ineligible to receive the full-dose cisplatinum-based chemotherapy
- POUT (Lancet 2020)
- Intravesical
- Adjuvant radiation
- Follow-up
- The primary aims of postoperative surveillance for UTUC are to identify urothelial recurrences, de novo tumours of the urinary tract, and distant metastases at early stages when they may be amenable to treatment
- Post-nephroureterectomy recurrences of the
- Retroperitoneum or pelvis occurr in ≈5%
- Contralateral upper tract occurred in ≈2%
- Port-site occur very infrequently, usually associated with inadvertent entry into the collecting system.
- Distant metastases
- Occurred following nephroureterectomy in 16% of patients.
- Median time to metastases was 13-16 months.
- The reported sites of metastases included retroperitoneal lymph nodes in 5%, lung in 5%, bone in 4% and liver in 4%. Less common sites included brain, adrenal gland and non-regional lymph nodes.
- The CUA surveillance protocol is based on pathologic tumour stage, pathological nodal status, grade, lymphovascular invasion status, and multifocality
- Post-operative evaluation must routinely include evaluation of the:
- Bladder
- Ipsilateral (if organ-sparing therapy was chosen) and contralateral urinary tracts
- Extra-urinary sites for local and metastatic spread
- Bladder
- Should be assessed with cytology and cystoscopy in all patients at months 3, 6, 12, 18, 24 and annually thereafter for up to 10 years of recurrence-free survival
- Ipsilateral upper tract
- Should be assessed by URS and selective cytology or biopsy in all patients following nephron-sparing procedures at months 3, 6, 12, 18, 24 and annually thereafter up to 10 years of recurrence-free survival
- Most patients following nephron-sparing procedures will develop ipsilateral upper tract recurrences.
- Ipsilateral upper tract tumor usually occurs in a proximal-to-distal direction; recurrence proximal to the original lesion is rare.
- This high rate of ipsilateral recurrence results in part from a multifocal field change, which is even more pronounced than in bladder cancer
- Computed tomography urography (CTU) and retrograde pyelography lacks sensitivity
- Most patients following nephron-sparing procedures will develop ipsilateral upper tract recurrences.
- Should be assessed by URS and selective cytology or biopsy in all patients following nephron-sparing procedures at months 3, 6, 12, 18, 24 and annually thereafter up to 10 years of recurrence-free survival
- Extra-urinary sites
- Routine blood work should include:
- Renal function tests
- Metabolic panel, including liver function tests, calcium and alkaline phosphatase
- To assess for local, contralateral and distant metastases in patients after nephroureterectomy or nephron-sparing procedures, imaging of the abdomen and pelvis with CTU is recommended
- MRI or US may be substituted for CTU in patients with contraindications to CTU
- CXR is recommended to assess for lung metastases
- Bone scan is indicated in the presence of bone pain, elevated calcium or elevated alkaline phosphatase to assess for bone metastases
- Routine blood work should include:
- Lack of evidence for an optimal duration of surveillance; recommended approach:
- High-grade, pT≥2 or pN+: lifelong annual surveillance with history, physical examination, blood work, urine cytology and abdominal/chest imaging
- Low-grade, pT<2 pN0/x: annual cystoscopy and ipsilateral ureteroscopy (following nephron-sparing procedures) may be omitted after 10 years of recurrence-free survival; after 10 years of recurrence-free survival, patients with may be discharged from annual surveillance
- After nephron-sparing therapy
- Metastatic disease
- Limited data on the efficacy of chemotherapy in metastatic UTUC
- Prospective randomized trials comparing chemotherapeutic regimens for UTUC are not feasible owing to the rarity of these patients.
- Regimen: MVAC (methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin)
- Continues to have the highest response rate
- Carboplatin is frequently substituted instead of cisplatin because of either limitations of renal function or concerns over nephrotoxicity associated with cisplatin. Results with carboplatin are inferior than cisplatin.
- Complete responses are rare in the metastatic setting, and the duration of response is limited, with overall survival of 12-24 months
- Limited data on the efficacy of chemotherapy in metastatic UTUC
Questions
- What are the risk factors for upper tract urothelial carcinoma (UTUC)?
- Which other malignancies are associated with Lynch syndrome?
- In patients with bladder cancer, what is the risk of subsequent UTUC? In patients with UTUC, what is the risk of subsequent bladder cancer?
- in patients that have undergone cystectomy for bladder cancer, which of the following is not a risk factor for subsequent UTUC?
- What is the most important predictor of developing metastasis?
- What is the differential diagnosis of a filling defect in the collecting system?
- What is the most important prognostic factor for survival in UTUC?
- What are the indications for nephroureterectomy? Segmental ureterectomy?
Answers
- What are the risk factors for upper tract urothelial carcinoma (UTUC)?
- Smoking
- Occupational exposure
- Chronic inflammation
- Cyclophosphamide exposure
- Analgesic abuse
- Lynch syndrome
- Exposure to aristolocholic acid (Balkan nephropathy)
- Exposure to arsenic
- Which other malignancies are associated with Lynch syndrome?
- Colonic (most common), endometrial (second most common), prostate, urothelial, adrenal, gastric, pancreatic, uterine, ovarian, and sebaceous carcinomas
- In patients with bladder cancer, what is the risk of subsequent UTUC? In patients with UTUC, what is the risk of subsequent bladder cancer?
- 2-4% patients with bladder cancer will subsequently develop UTUC,
- ≈30% patients with UTUC will subsequently develop bladder cancer after nephroureterectomy or nephron-sparing procedures
- In patients that have undergone cystectomy for bladder cancer, which of the following is not a risk factor for subsequent UTUC?
- Presence of CIS
- N0 status
- High-grade tumours
- Involvement of male prostatic urethra or female urethra
- What is the most important predictor of developing metastasis?
- T stage > 2
- What is the differential diagnosis of a filling defect in the collecting system?
- Tumour
- Blood clot
- Stone
- Sloughed papilla
- Fungus ball
- Overlying bowel gas
- External compression
- What is the most important prognostic factor for survival in UTUC?
- Stage; recall in bladder grade has stronger association with progression of NMIBC than stage
- What are the indications for nephroureterectomy? Segmental ureterectomy?
- Nephroureterectomy: high-grade or pT2
- Segmental ureterectomy:
- Low-grade, low-stage tumors that are not able to be removed endoscopically because of tumor size or multiplicity
- High-grade or invasive tumors when preservation of renal unit is necessary
Additional References
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, vol 1, chap 58