Kidney Cancer: Diagnosis and Evaluation
Jump to navigation
Jump to search
See 2021 AUA Renal Mass and Localized RCC Guideline Notes
Includes 2013 CUA Guidelines on Genetic Screening for Hereditary Renal Cell Cancers and parts from 2014 CUA Surgical Management of Renal Cell Carcinoma Consensus Statement and 2021 AUA Renal Mass and Localized RCC Guidelines
UrologySchool.com Summary[edit | edit source]
- Diagnosis and Evaluation in a patients with suspected renal cell carcinoma
- History and physical exam
- Labs:
- AUA (5):
- CBC
- Urinalysis (including assessment of proteinuria)
- Serum electrolytes
- Liver function tests
- Assessment of GFR
- CUA: CBC, Cr, AST/ALT (markers of liver dysfunction), ALP, corrected calcium (markers of bone dysfunction)
- Urine cytology in central tumours
- Markers of prognosis in advanced disease (Motzer/Heng criteria, see Advanced Kidney Cancer Chapter Notes)
- AUA (5):
- Imaging:
- Primary tumour
- AUA:
- Multiphase CT or MRI
- CUA:
- Triphasic CT abdomen/pelvis
- MRI if CT contraindicated (pregnant/allergy/CKD)
- If thrombus present, consider doppler US/MRI to stage IVC involvement
- Triphasic CT abdomen/pelvis
- AUA:
- Metastasis:
- AUA:
- CXR
- Indications for CT chest (3):
- Pulmonary symptoms
- Abnormal CXR
- High-risk disease, defined by (5):
- Presence of thrombi
- Presumed adenopathy
- Larger tumor size
- Infiltrative appearance
- Extensive tumor necrosis
- Indications for CT chest (3):
- CXR
- CUA:
- CXR, consider CT chest if ≥stage T2
- Bone scan, if clinically indicated or elevated alkaline phosphatase and serum calcium
- Brain CT or MRI if large volume metastatic disease
- AUA:
- Primary tumour
- Other:
- Renal mass biopsy
- Genetic counseling, if indicated
- Referral to nephrology, if indicated
History and Physical Exam[edit | edit source]
History[edit | edit source]
- Risk factors for RCC: smoking, hypertension, obesity, familial syndromes, CKD
- Family history
- Symptoms
- Most (>50%) of renal masses are diagnosed incidentally during an evaluation for unrelated signs or symptoms.
- Many remain asymptomatic and nonpalpable until they are locally advanced
- Flank pain is usually due to hemorrhage and clot obstruction, but can also occur with locally advanced or invasive disease.
- The “classic triad” of symptoms associated with a malignant renal mass was hematuria, flank pain and abdominal mass
- <5% of patients in contemporary series present with these symptoms
- Symptoms of advanced disease:
- Flank pain
- Locally advanced RCC usually presents with pain, generally from invasion of the posterior abdominal wall, nerve roots, or paraspinous muscles.
- Gross hematuria
- Constitutional symptoms such as weight loss, fever, and night sweats
- Flank pain
- New onset coughing or other respiratory issues may suggest pulmonary metastases
- New neurologic symptoms may suggest cerebral metastases
- Many remain asymptomatic and nonpalpable until they are locally advanced
- Most (>50%) of renal masses are diagnosed incidentally during an evaluation for unrelated signs or symptoms.
- Spontaneous perirenal hemorrhage is an uncommon presentation of RCC in which the underlying mass may be obscured. Repeat CT a few months later can provide a definitive diagnosis.
Physical Examination[edit | edit source]
- General
- Blood pressure
- Performance status
- Body habitus
- Dermatologic lesions, which may suggest a familial RCC syndrome
- Lymphadenopathy
- Cervical/supraclavicular
- Axillary
- Groin
- Abdomen
- Prior abdominal scars
- Genitals
- Scrotum
- Varicocele
- Scrotum
- Extremities
- Lower extremity edema
- Neurologic exam
- Should be performed if there is any suggestion of cerebral or spinal metastases
- Limited role in clinically localized disease
- Physical exam findings suggestive of advanced disease (5):
- Palpable abdominal mass
- Nonreducing varicocele
- Right-sided varicocele
- Bilateral lower extremity edema resulting from venous involvement
- Palpable cervical lymphadenopathy
Paraneoplastic syndromes in RCC[edit | edit source]
- Found in 10-20% of patients with metastatic RCC (NEW-HALF-CAP):
- Neuropathy (3%)
- Elevated ESR (56%, most common)
- Weight loss (34%)
- Hypertension (38%)
- Can be due to elevated renin production by tumour, compression of renal artery, or AV fistula in tumour
- Anemia (36%)
- Elevated LFTs (14%)
- Due to nonmetastatic hepatic dysfunction, also known as Stauffer syndrome
- Fever (17%)
- HyperCalcemia (5%)
- Can be due to (2):
- Paraneoplastic phenomena (production of PTH-related protein)
- Osteolytic metastatic involvement of the bone
- Signs and symptoms include nausea, anorexia, fatigue, and decreased deep tendon reflexes
- Management includes vigorous hydration followed by diuresis with furosemide and the selective use of bisphosphonates (if adequate renal function), corticosteroids or calcitonin
- Can be due to (2):
- Amyloidosis (2%)
- Polycythemia (4%)
- Management
- Treatment of paraneoplastic syndromes has required surgical excision or systemic therapy and, except for hypercalcemia, medical therapies have not proved helpful.
Labs[edit | edit source]
- There are no biomarkers or routine laboratory tests used to diagnose renal malignancies.
Recommended laboratory investigations in a patient with suspected RCC[edit | edit source]
AUA[edit | edit source]
- 2021 AUA Guidelines on Renal Mass and Localized Renal Cancer
- CBC
- Urinalysis (including assessment of proteinuria)
- Comprehensive metabolic panel (electrolytes, liver function tests, assessment of GFR)
- CBC should be considered prior to any intervention
- Urinalysis with dipstick and microscopic evaluation should be obtained to assess for proteinuria, hematuria, pyuria or signs of other genitourinary maladies.
- Presence of proteinuria is an important prognostic indicator and can be detected by standard urine dipstick.
- Patients with a positive dipstick test (1+ or greater) should undergo confirmation by a quantitative measurement (protein-to-creatinine ratio or albumin-to-creatinine ratio), as part of a focused medical workup for renal dysfunction.
- Microscopic hematuria should also be further assessed to rule out a co-existing urinary tract conditions
- Presence of proteinuria is an important prognostic indicator and can be detected by standard urine dipstick.
- The comprehensive metabolic panel should be reviewed for electrolyte abnormalities and hepatic functional parameters.
- Abnormalities in hepatic function may prompt further workup to exclude co-existing hepatic disease or metastases which may impact management or overall prognosis
- Elevated ALP and/or bone pain should prompt investigation of potential bone metastases
- GFR and degree of proteinuria should be used assign CKD stage in patients with a solid or Bosniak 3/4 complex cystic renal mass, as this will influence management
- Proper classification of CKD is outlined in the Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines, which takes into account (3):
- GFR (CKD-EPI GFR equation)
- Proteinuria
- Etiology of CKD
- Indications for referral to nephrology in a patient with a renal mass (4):
- Estimated GFR < 45 mL/min/1.73m2
- Confirmed proteinuria
- Diabetics with pre-existing CKD
- When eGFR is expected to be <30 mL/min/1.73m2 after intervention
- Proper classification of CKD is outlined in the Kidney Disease: Improving Global Outcomes (KDIGO) Guidelines, which takes into account (3):
CUA[edit | edit source]
- 2014 CUA Surgical Management of Renal Cell Carcinoma Consensus Statement (4):
- CBC
- Cr
- Markers of liver dysfunction (transaminases)
- Markers of bone dysfunction (ALP and corrected calcium)
Imaging[edit | edit source]
Primary[edit | edit source]
- Radiologic classification of renal masses
- Cystic
- Simple vs. Complex
- See Benign Renal Tumours Chapter Notes
- Simple vs. Complex
- Solid
- Differential diagnosis for radiographically detected solid renal mass:
- RCC
- Oncocytoma
- Angiomyolipoma
- Urothelial carcinoma
- Metastasis
- Abscess
- Infarct
- Vascular malformation
- Renal pseudotumor
- The diagnosis of most of these lesions can be established on the basis of clinical presentation and radiographic features, occasionally combined with endourologic studies or renal mass biopsy. However, with the exception of fat-containing AML, it is often not possible to reliably distinguish RCC from benign renal neoplasms, including oncocytoma and fat-poor AML, with current diagnostic techniques.
- Differential Diagnoses of Infiltrative Masses in the Kidney (7):
- Lymphoma
- High-grade urothelial carcinoma
- Sarcomatoid differentiation (chromophobe is the most prone one to have this)
- Collecting duct carcinoma
- Renal medullary carcinoma
- Xanthogranulomatous pyelonephritis (XGP)
- Metastasis (occasionally)
- Differential diagnosis for radiographically detected solid renal mass:
- Cystic
- Goals of imaging are to evaluate (4):
- Renal mass characteristics
- Tumor size
- Size is inversely correlated with risk of malignancy
- Proportion of surgically resected masses found to be benign[1]
- <2cm: 25%
- <4cm: 19%
- Proportion of surgically resected masses found to be benign[1]
- Size is inversely correlated with risk of malignancy
- Tumor complexity
- Degree of contrast enhancement
- Presence or absence of fat
- Involvement of or juxtaposition to the renal hilum, vein, or collecting system
- Tumor size
- Contralateral kidney
- Regional lymphadenopathy
- Adrenal involvement
- Findings suggestive of ipsilateral adrenal involvement (3):
- Enlarged or indistinct adrenal gland on imaging
- Extensive malignant replacement of the kidney
- Palpably abnormal adrenal gland
- Findings suggestive of ipsilateral adrenal involvement (3):
- Renal mass characteristics
Modalities[edit | edit source]
Options[edit | edit source]
- Ultrasound
- CT
- MRI
- Other
- Contrast-enhanced US
- PET-CT
- None of the current imaging modalities can reliably distinguish between benign and malignant tumors or between indolent and aggressive tumor biology
US[edit | edit source]
- Strict US criteria for a simple cyst (3):
- Smooth cyst wall
- Round or oval shape without internal echoes
- Through-transmission with strong acoustic shadows posteriorly
- Acute focal pyelonephritis (lobar nephronia) is hypoechoic
- Angiomyolipomas are usually echogenic
- RCCs and intrarenal abscesses have variable echogenicity
- Angiomyolipomas demonstrate a speed-propagation artifact
- Speed of sound in the fat is significantly slower than that in the soft tissue, due to the presence of fat in the tumor
- A renal mass that is not clearly a simple cyst by strict US criteria should be evaluated further with CT
CT[edit | edit source]
- Contrast-enhanced CT is the modality of choice in evaluating cystic renal masses.[2]
- MRI is used when CT is contraindicated (e.g., patients with allergy to iodinated contrast agent) or as a problem-solving modality for equivocal findings. MRI can show some septa that are less apparent at CT and demonstrate definitive enhancement in those cysts that show only equivocal enhancement at CT. As a consequence, renal cysts can be placed in a higher Bosniak category with MRI than with CT[3]
IV contrast[edit | edit source]
- Contraindications (2):
- Severe allergy
- Severe chronic kidney disease
- Patients with GFR <45 ml/min/1.73m2 should receive contrast with caution
- Patients with acute kidney injury or GFR < 30 mL/min/1.73m2 who are not undergoing renal replacement therapy should receive intravenous normal saline prophylaxis prior to receiving iodinated contrast media§.
- Patients with GFR 30-44 mL/min/1.73m2 may be considered for intravenous fluid prophylaxis per individual physician discretion based on the patient’s risk factor for renal injury§.
- MRI with second generation gadolinium-based intravenous contrast is now a safer option in many patients with severe CKD
- In patients who cannot receive intravenous contrast, MRI, non-contrast CT, and US (with Doppler) can be used to characterize renal masses
- Contrast-induced nephropathy
- Due to intrarenal vasoconstriction and tubular necrosis
- Risk factors
- Diabetes mellitus
- Advanced age
- Congestive heart failure
- Hypertension
- Dehydration
- Diuretic use
- Low hematocrit
- Ventricular ejection fraction < 40%
- Concomitant exposure to chemotherapy, aminoglycoside or nonsteroidal anti-inflammatory agents
- Hyperuricemia
- Diseases that affect renal hemodynamics, such as end-stage liver disease and nephrotic syndrome
- Patients with a diagnosis of a paraproteinemia syndrome/disease (e.g., multiple myeloma), history of a kidney transplant, renal tumor, renal surgery, or single kidney may also be at higher risk
- The patients at highest risk for developing CIN are those with both diabetes and pre-existing renal insufficiency.
- Metformin
- Patients with type 2 diabetes mellitus receiving metformin may have an accumulation of the drug after administering intravascular radiologic contrast medium (IRCM), resulting in biguanide lactic acidosis
- Biguanide lactic acidosis
- Symptoms of include vomiting, diarrhea, and somnolence
- Fatal in ≈50% of cases
- Rare in patients with normal renal function (no defined threshold but some studies suggest <60).
- In patients with normal renal function and no known comorbidities there is no need to discontinue metformin before IRCM use, nor is there a need to check creatinine following the imaging study.
- In patients with renal insufficiency metformin should be discontinued the day of the study and withheld for 48 hours. Post-procedure creatinine should be measured at 48 hours and metformin started once kidney function is normal.
- It is not necessary to discontinue metformin before gadolinium-enhanced MRI studies when the amount of gadolinium administered is in the usual dosage range of 0.1 to 0.3 mmol per kilogram of body weight.
- Prevention of CIN is of great concern and has been a subject of many different studies. Hydration is the major preventative action against CIN. Periprocedural IV hydration with 0.9% saline at 100 mL/hr 12 hours before to 12 hours after has been shown to decrease the incidence of CIN after IV contrast use
Findings[edit | edit source]
- Enhancement
- Hounsfield units (HU) are a standardized quantitative measurement of x-ray attenuation[4]
- If homogenous lesion and HU on non-contrast CT is[5]
- <20, then simple cyst
- >70, then hemorrhagic/proteinaceous cysts
- 20-70, then considered indeterminate and warrants further evaluation
- On contrast-enhanced CT, if change in HU (compared to non-contrast)[6]
- >20, then considered enhancing
- <10, then no enhancement
- 10-20, then indeterminate enhancement
- Differential diagnosis of an enhancing renal mass on CT scan[7]
- Hyperdense cyst
- Hyperdense cysts are benign lesions that contain old, degenerated, or clotted blood and have increased CT attenuation (>20 HU)
- Renal cell carcinoma
- Any solid renal mass that enhances >15 Hounsfield units and does not exhibit fat density should be considered a renal cell carcinoma (RCC) until proven otherwise
- Clear cell enhances more than papillary and chromophobe RCC
- Emerging data suggests that clear cell RCC may be distinguished from the papillary subtype (papillary RCC is often hypo-enhancing). However, both malignant and benign masses can display heterogeneous avid contrast enhancement patterns and no definitive conclusion can be drawn regarding biological potential based on enhancement pattern alone
- Clear cell enhances more than papillary and chromophobe RCC
- Any solid renal mass that enhances >15 Hounsfield units and does not exhibit fat density should be considered a renal cell carcinoma (RCC) until proven otherwise
- Hyperdense cyst
- Solid masses that have substantial areas of negative CT attenuation (<-20 HU) indicative of fat are diagnostic of AML
- ≈5-10% of AML’s are fat poor
- In rare instances RCC may demonstrate fat density on imaging and even pathologically, but this is the exception rather than the rule
- Tumors with calcification associated with fat are uncommon but are almost always malignant RCC.
- In this setting the fat is thought to be a reactive process related to tumor necrosis.
- Calcification is virtually never seen in association with AML.
- Lymphadenopathy
- Enlarged hilar or retroperitoneal lymph nodes (≥2 cm) on CT almost always harbor malignancy, but this should be confirmed by surgical exploration or percutaneous biopsy if the patient is not a surgical candidate.
- Many smaller nodes prove to be inflammatory rather than neoplastic and should not preclude surgical therapy
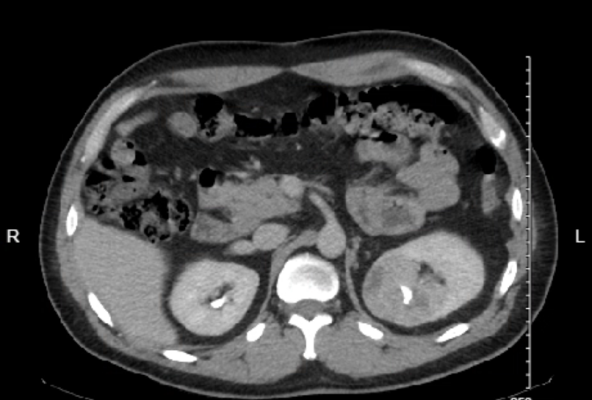
|
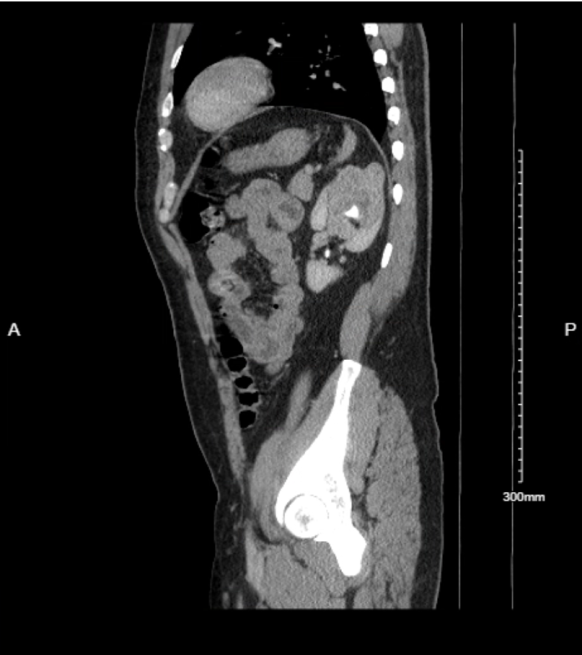 |
|---|---|
| Axial view | Sagittal view |
MRI[edit | edit source]
- Alternate standard to CT
- Similar sensitivity and specificity to CT
- Sensitivity: 88% (interquartile range [IQR] 81%-94%) CT vs. 87.5% (IQR 75.25%-100%) MRI
- Specificity: 75% (IQR 51%-90%) CT vs. 89% (IQR 75%-96%) MRI
- Vogel, Christina, et al. "Imaging in suspected renal-cell carcinoma: systematic review." Clinical genitourinary cancer 17.2 (2019): e345-e355.
- Considered comparable to CT in characterizing indeterminate renal masses by the American College of Radiology[8]
- CT may be better for smaller lesions
- Most useful in patients in whom contrast is contraindicated because of severe allergy or severe CKD
- Gadolinium contrast can be given to patients with GFR < 30 mL/min/1.73m2[9]
- Nephrogenic systemic fibrosis (NSF)
- Fibrosing dermopathy associated with soft tissue deposition and accumulation of gadolinium
- Potentially serious complication of gadolinium contrast
- Very rare
- More common with group I gadolinium based contrast agents
- Incidence <0.07% in patients with CKD 4 and 5 with group II agents
- Group II gadolinium based contrast agents are considered safe for any level of eGFR
- Renal function does not need be screened prior to receiving group II gadolinium based contrast agents
- More common with group I gadolinium based contrast agents
- Prevention in patients with ESRD: perform hemodialysis after the MRI scan.
- Similar sensitivity and specificity to CT
- Image Sequences
- Lesions in upper pole and lower pole may be skipped when scrolling through axial slices, always look at coronal images
- T2WI
- Most useful for anatomic assessment of renal masses
- Usually two T2 sequences, one with fat suppression and one without
- Renal vessels will be dark
- Diffusion weighted imaging
- Sequences with higher b-value more likely useful
- Enhancement > 20% is suspicious for RCC
- For a fat-containing tumor, a T2-weighted image with fat suppression is most likely to identify macroscopic fat and confirm the diagnosis of an angiomyolipoma (AML).
- Best study for evaluation of invasion into adjacent structures
- Large tumours may indent and compress adjacent liver parenchyma but seldom actually grow by direct extension into the liver; obliteration of the fat plane between the tumour and adjacent organs (e.g. the liver) on CT can be a misleading finding and should prompt further imaging with MRI. In reality, surgical exploration is often required to make an absolute differentiation.
Contrast-enhanced US using microbubbles[edit | edit source]
- May play an important role in the future for characterizing renal masses in select patients in whom other forms of intravenous contrast are contraindicated.
RENAL nephrometery score[edit | edit source]
- Based on the 5 most reproducible features that characterize the anatomy of a solid renal mass
- (R)adius (maximal tumor size)
- (E)xophytic/endophytic
- (N)earness of the deepest portion of the tumor to the collecting system or renal sinus
- (A)nterior (a)/posterior (p) descriptor
- (L)ocation relative to the polar line
- All components except for the (A) descriptor are scored on a 1, 2, or 3-point scale.
- Range 4-12, lower number more amenable to partial nephrectomy:
- More details on RENAL nephrometery score
Metastasis Staging[edit | edit source]
- Chest x-ray in patients with suspected renal malignancy
- Not indicated in patients with suspected or confirmed benign renal masses
- Chest is most common site of visceral metastasis
- Indications for CT chest (3):[10]
- Pulmonary symptoms
- Abnormal CXR
- High-risk disease, defined by (5):
- Presence of thrombi
- Presumed adenopathy
- Larger tumor size
- Infiltrative appearance
- Extensive tumor necrosis
- Bone scan
- Should be reserved primarily for patients with bone pain or elevated alkaline phosphatase
- Brain imaging for those with neurologic symptoms
- Positron emission tomography (PET)
- Has been investigated for patients with high risk of metastatic RCC, with most studies showing good specificity but suboptimal sensitivity.
- Currently, PET scan has no role in the routine evaluation or staging of RCC.
Other investigations[edit | edit source]
Renal mass biopsy[edit | edit source]
Rationale[edit | edit source]
- ≈20-30% of clinically localized solid, enhancing, clinical T1 renal masses are benign
- The smaller the tumour size, the more likely to be benign.
- Risk of malignancy increases 30%/cm increase in tumour size
- Young to middle-age women, in particular, are more likely to have benign pathology, as high as 40% in some series.
- Some benign renal masses, such as cystic nephroma and atypical AML, may be influenced by the hormonal milieu and are thus more common in women
- Most benign clinical T1 renal masses are oncocytomas or atypical AMLs.
- The smaller the tumour size, the more likely to be benign.
Test performance characteristics[edit | edit source]
- A diagnosis of malignancy or RCC on RMB is highly reliable
- Mean diagnostic rate: 92% (8% non-diagnostic rate)[11]
- Pooled sensitivity: 96.7%
- Pooled specificity: 94.4%
- Pooled positive predictive value: 98.8%
- Pooled negative predictive value: 80.8%
- Histologic concordance: 90%[12]
- Grade concordance: 62%[13]
- PET scanning coupled with administration of radioactively labeled anti–CA-IX monoclonal antibody has been reported as an alternative to RMB
Limitations (4)[edit | edit source]
- A benign biopsy must be distinguished from a non-diagnostic biopsy (renal parenchyma or connective tissues) result.
- Non-diagnostic rate of renal mass biopsy is approximately 14%, which can be substantially reduced with repeat biopsy
- A benign biopsy may not always correlate with benign histology.
- Due to the imperfect nature of renal mass biopsy, patients with benign renal mass biopsy may warrant follow-up.
- Grade concordance from biopsy to surgically resected tissue is imperfect.
- Oncocytic neoplasms may represent a diagnostic dilemma.
Indications[edit | edit source]
CUA[edit | edit source]
- 2022 CUA Guidelines on Management of Small Renal Masses
- Should be offered when the result of the biopsy will influence management
AUA[edit | edit source]
- 2021 AUA Guidelines on Renal Mass and Localized Renal Cancer
- Currently has an adjunctive role in the diagnosis and risk stratification of patients with renal masses suspicious for RCC
- Consider biopsy when a mass is suspected to be hematologic, metastatic, inflammatory, or infectious.
- See Kidney Cancer: Non-Renal Cell Carcinoma Renal Malignancies Chapter Notes
- If metastatic cancer is confirmed, systemic treatment is typically prioritized.
- Index of suspicion for a non-neoplastic process, such as renal sarcoidosis, abscess, or focal pyelonephritis, should be increased in patients presenting with signs and symptoms consistent with an infectious or inflammatory condition or those with a prior history of recurrent infections or autoimmune disease
- Should be obtained if it will influence management
- NOT required for (2):
- Young or healthy patients who are unwilling to accept the uncertainties associated with RMB
- Older or frail patients who will be managed conservatively independent of RMB findings
- NOT required for (2):
Contraindications[edit | edit source]
- Biopsy or aspiration of cystic renal masses is generally not recommended, unless there is a targetable solid component, due to (2):[14]***#Concerns regarding tumor spillage
- High likelihood of obtaining a non-informative result due to sampling error
Technique[edit | edit source]
- May be performed under CT or US guidance
- Multiple core biopsies are preferred over fine needle aspiration[15]
- At least 2-3 cores being obtained with a 16-18 gauge needle to optimize diagnostic yield
Complications[edit | edit source]
- Overall complication rate: 8%[16]
- Vast majority of these complications reported as Clavien-Dindo <2 (>99%)
- Most common (5):
- Renal hematoma (4.9%)
- Clinically significant pain (1.2%)
- Gross hematuria (1.0%)
- Pneumothorax (0.6%)
- Hemorrhage requiring transfusion (0.4%)
- No reported cases of tumor seeding using contemporary techniques§
Genetic counseling[edit | edit source]
- Benefits (2):
- May allow for more intensive evaluation of the patient for RCC and associated manifestations
- Identification of blood relatives that may be at syndromic risk
Indications[edit | edit source]
AUA[edit | edit source]
- 2021 AUA Guidelines on Renal Mass and Localized Renal Cancer (5):
- Age ≤ 46 years with renal malignancy
- Multifocal or bilateral renal masses
- Family history (first-or second-degree relative) with a history of renal malignancy
- Personal or family history suggests a familial RCC syndrome (even if kidney cancer has not been observed)
- Pathology demonstrates histologic findings suggestive of such a familial RCC syndrome
- Hybrid oncocytic/chromophobe tumors are suggestive of BHD
CUA[edit | edit source]
- 2013 CUA Guidelines on Genetic Screening for Hereditary Renal Cell Cancers
- Any renal tumour (benign or malignant) AND any one of the following:
- Bilaterality or multifocality
- Age of onset ≤45
- 1st or 2nd degree relative with any renal tumour
- Stigmata of RCC syndrome (8)
- History of pneumothorax* (*or 1st degree relative with same) (found in BHDS)
- Pulmonary lymphangiomyomatosis* (TSC)
- Childhood seizure disorder* (TSC)
- Skin leiomyomas* (HLRCC)
- Skin fibrofolliculomas/ trichodisomas* (BHDS)
- Pheochromocytoma/ paraganglioma* (VHL)
- Hemangioblastoma of the retina, brainstem, cerebellum or spinal cord* (VHL)
- Early onset of multiple uterine fibroids (<30 years of age)* (HLRCC)
- Non-ccRCC with unusual associated features (e.g., chromophobe, oncocytic or hybrid tumours)
- Patients, with or without RCC, who report a family member (any) with any one of the following:
- Von Hippel-Lindau syndrome
- Hereditary papillary renal cell cancer
- Hereditary leiomyomatosis and renal cell cancer
- Birt-Hogg-Dubé syndrome
- Hereditary paraganglioma/ pheochromocytoma
- Tuberous sclerosis
- Any renal tumour (benign or malignant) AND any one of the following:
Stage at Diagnosis[edit | edit source]
- United States (SEER)[17]
- Localized: 68%
- Regional: 14%
- Distant: 13%
- Unstaged: 5%
Questions[edit | edit source]
- What is the differential diagnosis for a solid mass on imaging?
- What are the recommended investigations in patients with suspected RCC?
- What are the paraneoplastic syndromes associated with RCC?
- What findings are suggestive of ipsilateral adrenal involvement
- What are potential complications related to renal mass biopsy?
Answers[edit | edit source]
- What is the differential diagnosis for a solid mass on imaging?
- Malignant: RCC, urothelial carcinoma, sarcoma, lymphoma, metastasis
- Benign: oncocytoma, abscess, infarct, vascular malformation, renal pseudotumour
- What are the recommended investigations in patients with suspected RCC?
- History and physical
- Laboratory investigations: CBC, Cr, LFTs, calcium
- Imaging: Triphasic CT abdo/pelvis, CXR or CT chest
- What are the paraneoplastic syndromes associated with RCC?
- Neuropathy
- Elevated ESR
- Weight loss
- Hypertension
- Anemia
- LFTs elevated
- Fever
- Hypercalcemia
- Amyloidosis
- Polychythemia
- What findings are suggestive of ipsilateral adrenal involvement
- Enlarged or indistinct adrenal gland on imaging
- Extensive malignant replacement of kidney
- Palpably abnormal adrenal gland
- What are potential complications related to renal mass biopsy?
- Bleeding
- Renal hematoma
- Gross hematuria
- Hemorrhage requiring transfusions
- Pain
- Pneumothorax
- Bleeding
Next Chapter: Management of Localized and Locally Advanced Disease[edit | edit source]
References[edit | edit source]
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, chap 57
- Wein AJ, Kavoussi LR, Partin AW, Peters CA (eds): CAMPBELL-WALSH UROLOGY, ed 11. Philadelphia, Elsevier, 2015, chap 2
- Campbell, Steven, et al. "Renal mass and localized renal cancer: AUA guideline." The Journal of urology 198.3 (2017): 520-529.